Venus and Mars. Popular mythologies aside, neither planet has a particularly hospitable temperament. Both are rocky planets, like earth, and both have atmospheres of carbon dioxide (about 95%) and nitrogen (about 3%). Surface temperatures on Venus hover around 450oC; those on Mars about -53oC. Venus is not only a tad warm, its surface atmospheric pressure is 92 times that on earth; that on Mars about 6 one thousandths of earths comfort zone. Venus’ inclemency is the result of run-away Greenhouse processes; Mars’ is due to virtually no Greenhouse effects. Earth resides in that ‘Goldilocks zone’, being neither too hot nor too cold. Lucky us!
An energy budget
The sun heats the earth’s surface, atmosphere and oceans; earth’s internal heat contributes very little to this process. For the climate to be stable over a reasonable length of time (decades, centuries) there must be a balance between incoming heat from the sun and outgoing heat lost to space; this heat is lost via reflection, convection and conduction. Perturbations in this balance result in the surface either heating up or cooling down. We know that these long-term imbalances do occur because there have been glaciations and intervening periods of more clement surface conditions. Major climatic variations like these are mainly the result of (predictable) periodic perturbations in earth’s orbit and rotation, namely the Milankovitch cycles.
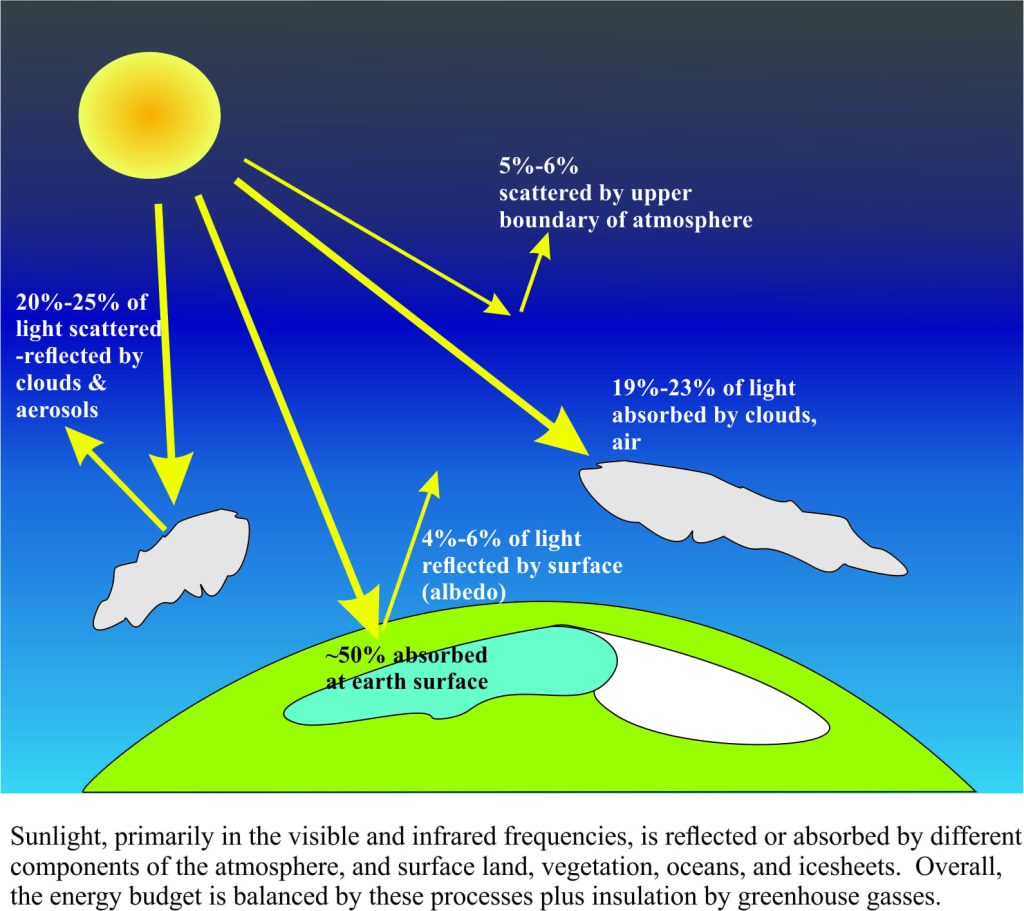
The total energy that earth receives from the sun is called the total radiance. It is primarily in the form of visible and infrared light, with lesser proportions of short wave-length UV, gamma and x-ray frequencies, and longer wavelength frequencies like microwaves. Partitioning of the incoming light is illustrated in the cartoon below. About 30% is reflected directly back to space by clouds, aerosols and the earth’s surface, especially ice sheets (referred to as the albedo); 70% is absorbed by the atmosphere, and both the solid and liquid earth.
 However, to maintain an energy balance, some of this absorbed light energy must be converted and re-radiated back to space. We witness this re-radiation in our everyday lives. The many hues of red, blue, green, and yellow in my backyard are visible because a part of the light spectrum is reflected. If the entire spectrum is reflected, we see white; the red flowers are reflecting only light in the red part of the spectrum – the remaining light energy is absorbed. The absorbed energy is absolutely necessary for biological growth. If no light or heat energy is reflected, we see nothing – black.
However, to maintain an energy balance, some of this absorbed light energy must be converted and re-radiated back to space. We witness this re-radiation in our everyday lives. The many hues of red, blue, green, and yellow in my backyard are visible because a part of the light spectrum is reflected. If the entire spectrum is reflected, we see white; the red flowers are reflecting only light in the red part of the spectrum – the remaining light energy is absorbed. The absorbed energy is absolutely necessary for biological growth. If no light or heat energy is reflected, we see nothing – black.
The greenhouse blanket
The Martian surface is frigid because most of the light-heat energy that gets to the planet’s surface is reflected and re-radiated back to space – Mars has no blanket. Our own atmosphere is made up of 78% nitrogen, 21% oxygen, and very small amounts of carbon dioxide, methane, and water vapour. Nitrogen and oxygen are almost completely transparent to sunlight in the visible and infrared part of the spectrum – they do little to help warm or cool the planet. That task has been appointed to the greenhouse gasses; water vapour (most abundant), carbon dioxide, methane, nitrous oxide, and more recently certain industrial hydrocarbons that have chlorine and fluorine in their molecular structure. Despite their very low concentrations in the atmosphere (water vapour is most abundant, CO2 is 0.04%, methane is even less), they are solely responsible for maintaining the kind of climatic conditions we have become accustomed to. All except the chlorofluorocarbons occur naturally.
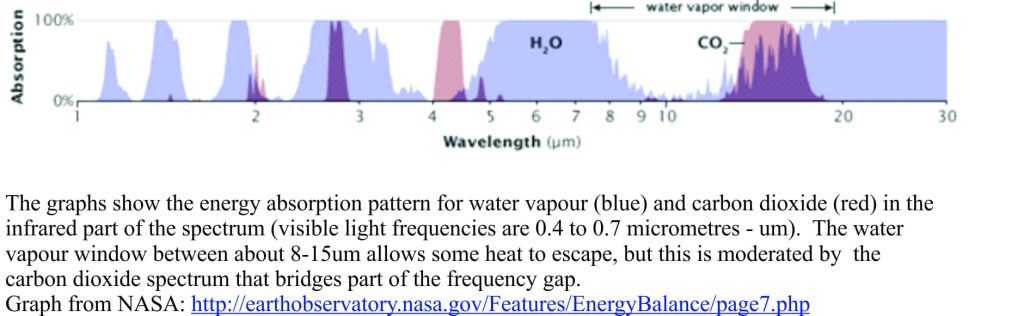
How does this greenhouse process work? As an example, a carbon dioxide molecule consists of a carbon atom bonded to two oxygen atoms. Bonding is loose enough such that infrared light energy will cause the atomic bonds to vibrate; in doing so the molecule absorbs heat. All the greenhouse gasses operate on the same principle. However, they also react to heat energy at different frequencies in the infrared part of the spectrum – this is illustrated below, comparing water and CO2. Water vapour absorbs energy over certain frequencies across the light spectrum, but importantly there is a ‘window’ between about 8-15 micrometres (part of the infrared frequency range) where it does not absorb heat; heat can escape through this frequency window.
This creates for us a wonderfully fortuitous balance between some infrared heat being absorbed by water vapour and other parts of the heat energy spectrum that can escape to space. Part of the energy balance is also moderated by CO2 that absorbs heat in parts of the infrared spectrum that water does not. Nitrous oxide and methane also play an important role in maintaining the balance between heat that is absorbed, and heat that is redirected to space. It is worth reiterating that, except for water, the concentrations of these greenhouse gasses are very low and that even minor changes to these amounts will result in some degree of warming or cooling of the atmosphere.
An additional factor in the greenhouse effect is the amount of aerosol and extremely fine particulate matter in the atmosphere. Volcanic eruptions contribute some of these although addition to the atmosphere is sporadic. The potential cooling effect from violent eruptions is well documented (Krakatoa 1883, Pinatubo 1991). Soot from industrial burning and clearing of forests is also present and may influence atmospheric heating.
Although oxygen has no role in greenhouse maintenance, it does interact with certain ultraviolet light frequencies to produce ozone (O3) in the upper atmosphere; this happy circumstance means that most of the harmful UV energy is filtered out by ozone before it reaches the surface.
Postnote
 There is an enduring image of Earth rising above the moon’s horizon, taken during the first manned lunar mission. Small, cloud swirled, seemingly fragile. Our atmosphere looks thin. When you look at this image, and then consider some of the details of how the atmosphere works, the balancing acts among all the gas components, the partitioning of heat and mass from air, earth and oceans, you realize how precarious the conditions conducive to our well-being really are. I’m not sure who said it first, but it really is all we’ve got.
There is an enduring image of Earth rising above the moon’s horizon, taken during the first manned lunar mission. Small, cloud swirled, seemingly fragile. Our atmosphere looks thin. When you look at this image, and then consider some of the details of how the atmosphere works, the balancing acts among all the gas components, the partitioning of heat and mass from air, earth and oceans, you realize how precarious the conditions conducive to our well-being really are. I’m not sure who said it first, but it really is all we’ve got.











1 thought on “The Greenhouse Advantage”
What ever we do we must stop the unbalance and try to reverse the pollution everyone is responsible to help in it to balance the earths atmosphere