A “Nitrate timebomb”. Last week’s media metaphor (Nov 10, 2017), was no doubt intended to create visions of dire deeds. After all, it seems that explosions are not in short supply these days. The actual story though is more droll, based as it is on the slow leakage of excess chemicals called nitrates, into the global environment. No fireworks; only leakage. The headline in several media outlets, only lasted a day or two, barely scratching our collective consciousness. Perhaps the problem is too big, or too remote – a candidate for the too-hard-basket. As Mark Twain might have said, “I guess so, I dunno”.
A “Nitrate timebomb”. Last week’s media metaphor (Nov 10, 2017), was no doubt intended to create visions of dire deeds. After all, it seems that explosions are not in short supply these days. The actual story though is more droll, based as it is on the slow leakage of excess chemicals called nitrates, into the global environment. No fireworks; only leakage. The headline in several media outlets, only lasted a day or two, barely scratching our collective consciousness. Perhaps the problem is too big, or too remote – a candidate for the too-hard-basket. As Mark Twain might have said, “I guess so, I dunno”.
Nitrogen itself is not a concern; every breath we take contains 80% N2. It’s what we do with nitrogen that is causing problems, particularly in natural systems like soils, surface waters, groundwater aquifers, and ultimately, the oceans. The scientific paper that caused these brief media conniptions was published this month in Nature Communications (it is Open Access).
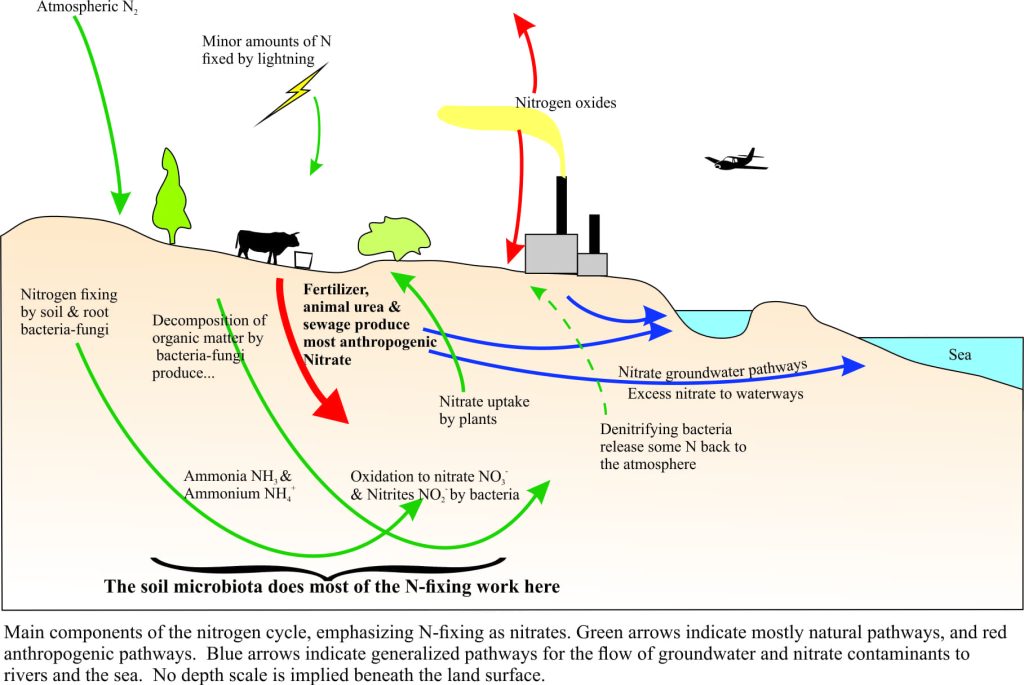
In natural earth systems, nitrogen and its chemically reactive derivatives such as ammonia (NH3), nitrates (NO3–), and nitrites (NO2–), participate in a grand cycle that, next to carbon, is absolutely critical to our well-being; nitrogen is involved in many biochemical reactions (for example all amino acids and proteins). The so-called nitrate timebomb exists because we have messed with this cycle. The cartoon below shows some of the loops, twists and turns that compounds of nitrogen make as they journey through various earth systems.
There are complex feedback processes among natural systems, like soils, vegetation, animals, water and so on, that account annually for the addition of about 210 million tonnes of reactive nitrogen. Most of this nitrogen is consumed naturally so that a reasonable balance is maintained. But our own activities, particularly combustion (industry, engines, and forests), sewage, fertilizer production, and agriculture, have almost doubled the amount of available reactive nitrogen added annually. Most of this additional nitrogen upsets the natural balance.
Plants do not get their nitrogen nutrition directly from air; instead they rely on nitrogen being transformed chemically to compounds that are soluble in water, some as ammonia, but predominantly as nitrates. The important drivers of nitrogen fixing are soil and plant-root microbiota, principally bacteria and fungi. Lightning also produces small amounts of reactive nitrogen. In natural systems, most soil fixing of nitrate keeps pace with plant uptake; there is little or no excess nitrate. Agriculture has taken advantage of vegetations’ predilection for nitrogen, by producing ever-increasing volumes of fertilizer. In most cases, the nitrogen is present as nitrate; it is a form immediately available for plants to use (as long as there is also water available). The application of fertilizers has been a major boon to global food production; fertilizer production has increased globally by a factor of 10 since 1950.
But increased food production has come at a price: huge tracts of the world’s soils have been seriously degraded, and and excess nitrate is adversely affecting other parts of the ecosystem. Too often, fertilizer is applied in quantities that ensures nitrate-nitrogen is available in-excess of a plant’s needs. However, the excess nitrate doesn’t just disappear – in fact it is stored in soils and subsoil materials (for months, years, decades), and eventually it is transported by gradual seepage into shallow groundwater aquifers and surface waterways (streams, lakes). Globally, nitrates are the most common groundwater contaminant.
Levels of nitrate contamination in soils and water are determined by several factors, including:
- Proximity to nitrate sources,
- The initial nitrate (fertilizer, sewage) input),
- The permeability of the soil and subsoil (i.e. the ease with which it will move through soil pores),
- Precipitation rates – high rainfall will tend to dilute excess nitrate,
- Irrigation will exacerbate nitrate leaching from surface applications to groundwater,
- The depth to the local watertable, and the rate at which groundwater flows through an aquifer before being intercepted by wells or freshwater-marine water bodies.
The World Health Organisation recommends no more than 10 milligrams per liter (10 mg/L) nitrate-nitrogen in drinking water, a value adopted by many countries. Shallow aquifer systems (less than 30-40m deep) tend to be most susceptible to nitrate (and other) contamination, particularly if agricultural practices occur in the same watershed (the USGS has released contaminant probability maps). Although there are significant regional differences, most aquifers in this category, on a global scale, have between 1-10 mg/L nitrates, and many exceed this value, some by 1 and 2 orders of magnitude. This includes aquifers in the USA and Europe, China, and India (there are many other examples).
Nitrate contamination in shallow aquifers will eventually find its way to surface water bodies such as rivers, lakes, and nearshore marine environments. Nitrate leaching (commonly in parallel with phosphates) is strongly implicated in promoting algal blooms in surface water, blooms that in many cases are toxic to marine and freshwater life, and people. Algal blooms also reduce the amount of dissolved oxygen in water, and in extreme circumstances can render water bodies biologically ‘dead’. The coastal province of Shandong, China, for example, is notorious for producing massive blooms of green marine algae, that most analysts consider result from agricultural and sewage effluent.
Not all phytoplankton blooms in oceans and lakes are caused by anthropogenic nitrates (and phosphates), but it seems that an increasing number of them are.
Nitrate in groundwater systems has been known for decades. However, it has taken longer for us (collectively) to realize that huge volumes of nitrate are being stored in subsoils between the surface and the watertable; the estimate quoted in the recent Nature Communications paper, indicates between 600 million and 1800 million tonnes of peak nitrate storage between 1900 and 2000. The concern is that this nitrate lies in-waiting. The rate of nitrate movement through subsoil materials is highly variable, but generally very slow – commonly on the order of decades. This means that, even if we were to immediately cut further increases in anthropogenic nitrate, many subsoil and aquifer systems would take decades to flush the offending contaminants. And therein lies the other conundrum – nitrates will continue to be flushed into our waterways for the foreseeable future.
The nitrate problem is gaining recognition. Its solution requires a rethink of established agricultural and waste disposal practices, of entrenched corporate attitudes that more (fertilizer) is always better, and the political will to work through the multitude of issues.
The problem is huge, but we need to make certain it is not put in the ‘too-hard basket’.