Pressure solution and stylolite formation in carbonates.
This is part of the How To…series on carbonate rocks
Pressure solution takes place in lithified rock when stress at intergranular and intercrystalline boundaries exceeds normal hydrostatic pressure. Differential stress can develop at almost any stage of sediment burial and compaction, during tectonic deformation and metamorphism. This post will describe pressure solution in carbonates, but it is worthwhile remembering that it can occur in many sediment types, as well as deformed rock. For example, cleavage, a penetrative fabric commonly found in folded metamorphosed strata, develops via a combination of shortening, pressure solution of certain mineral components and precipitation of new minerals. It is generally understood that pressure solution will occur if three conditions are met:
- Differential compressive stresses develop at intergranular contacts.
- Dissolved components are transported from the grain contacts to regions of lower compressive stress; this requires efficient fluid movement, and
- The solute reprecipitates some distance from its point of origin.
In sedimentary rocks, pressure solution is commonly manifested as:
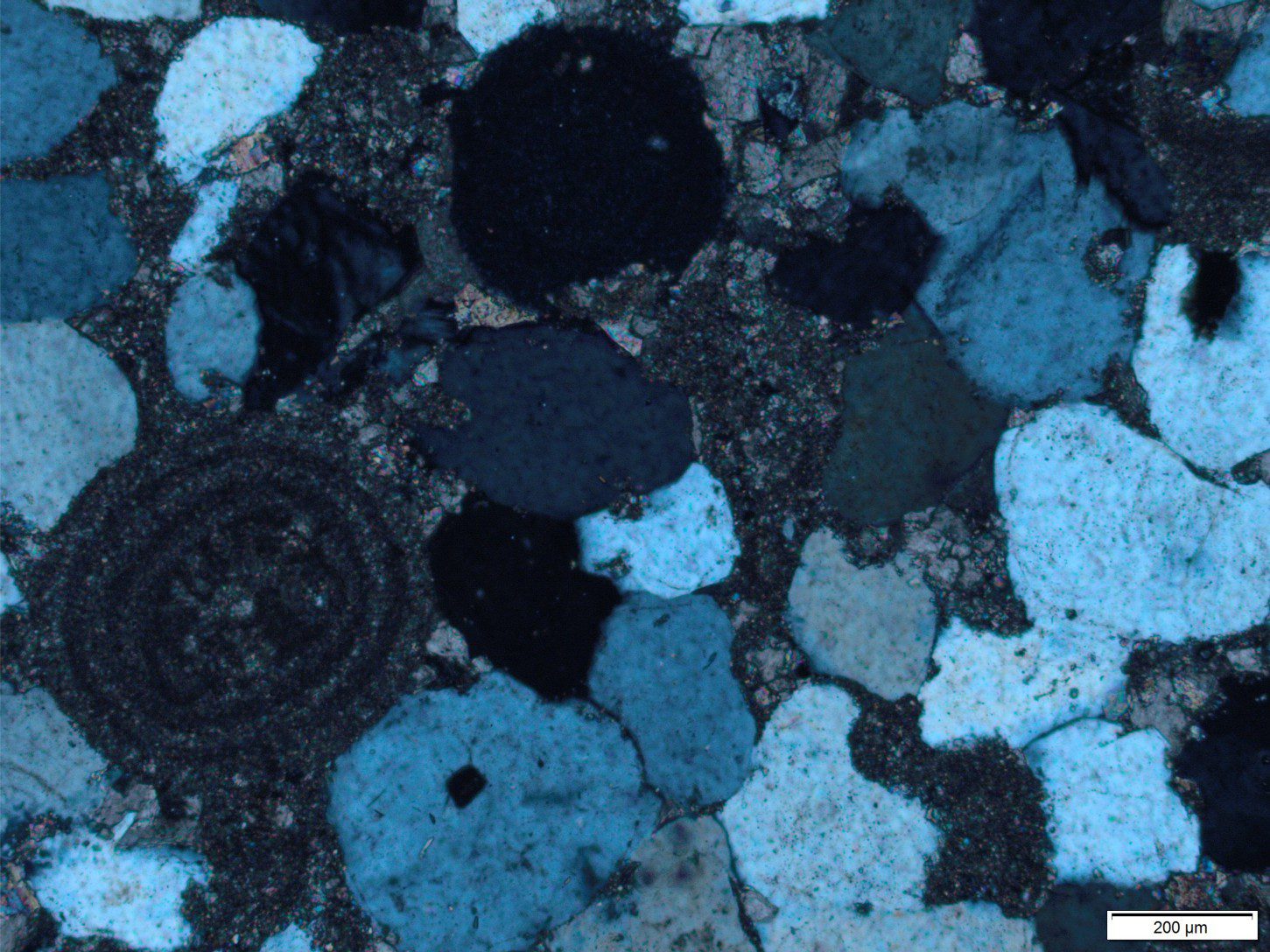
- Discontinuous grain-to-grain contacts, where one grain crosscuts or penetrates another grain. Intergranular pressure solution was first described by Henry Clifton Sorby in 1863. Pressure solution in ooid grainstones is a good example because we know the original shape of the grains involved. Intergranular pressure solution can develop between quartz grains but is a slower process than in carbonates because of its much lower solubility.
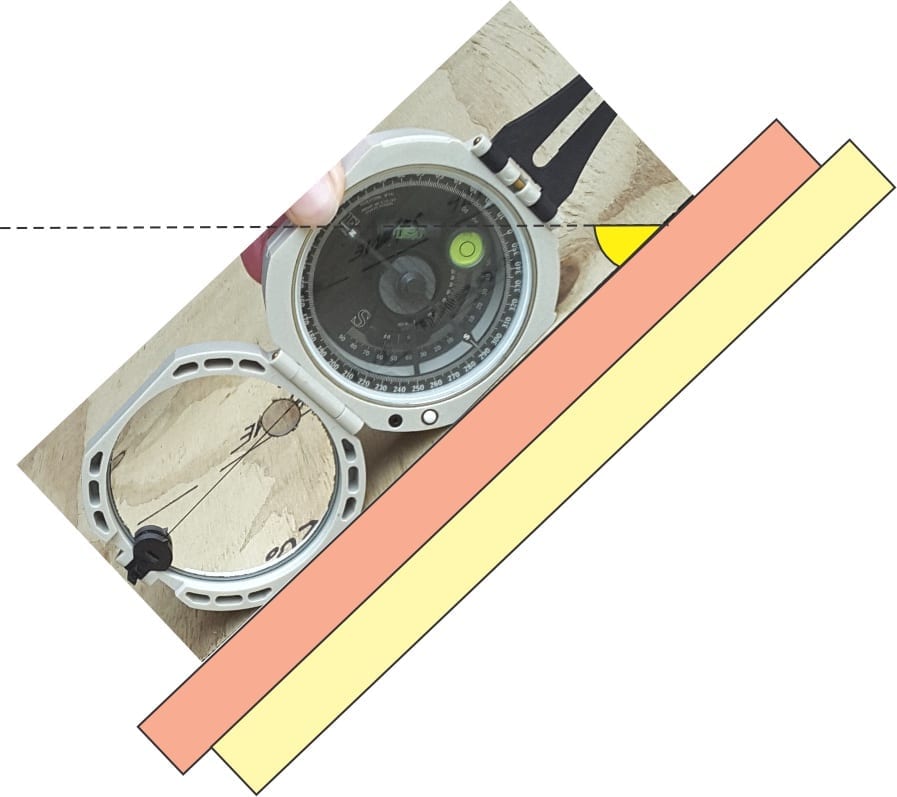
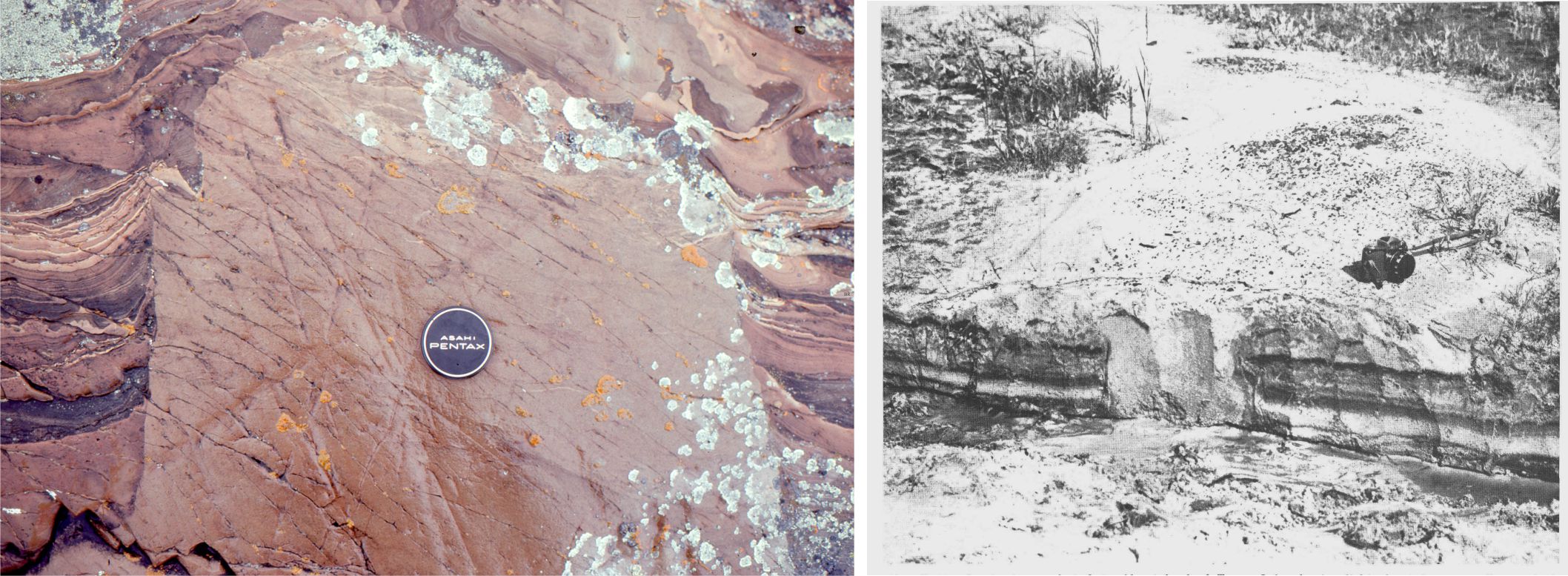
- One of the earliest descriptions of stylolites was presented by K.F. Klöden, a German ‘naturalist’ who in 1928 provided us with the name stylolite, but interpreted them as fossil traces. We now know that stylolites represent pressure solution across an extensive surface, producing contacts with complex sawtooth and interdigitate geometries. Surfaces can extend laterally many metres; the relief on a stylolite surface ranges from millimetres to 10s of centimetres. Stylolite surface orientation is determined by the principal compressive stress which is close to vertical if the stress is borne of sediment compaction – therefore, surfaces commonly parallel bedding but may diverge from bedding if tectonic deformation is involved. The presence of stylolites indicates dissolution of carbonate and a reduction in stratigraphic thickness; losses of 10% thickness are common.
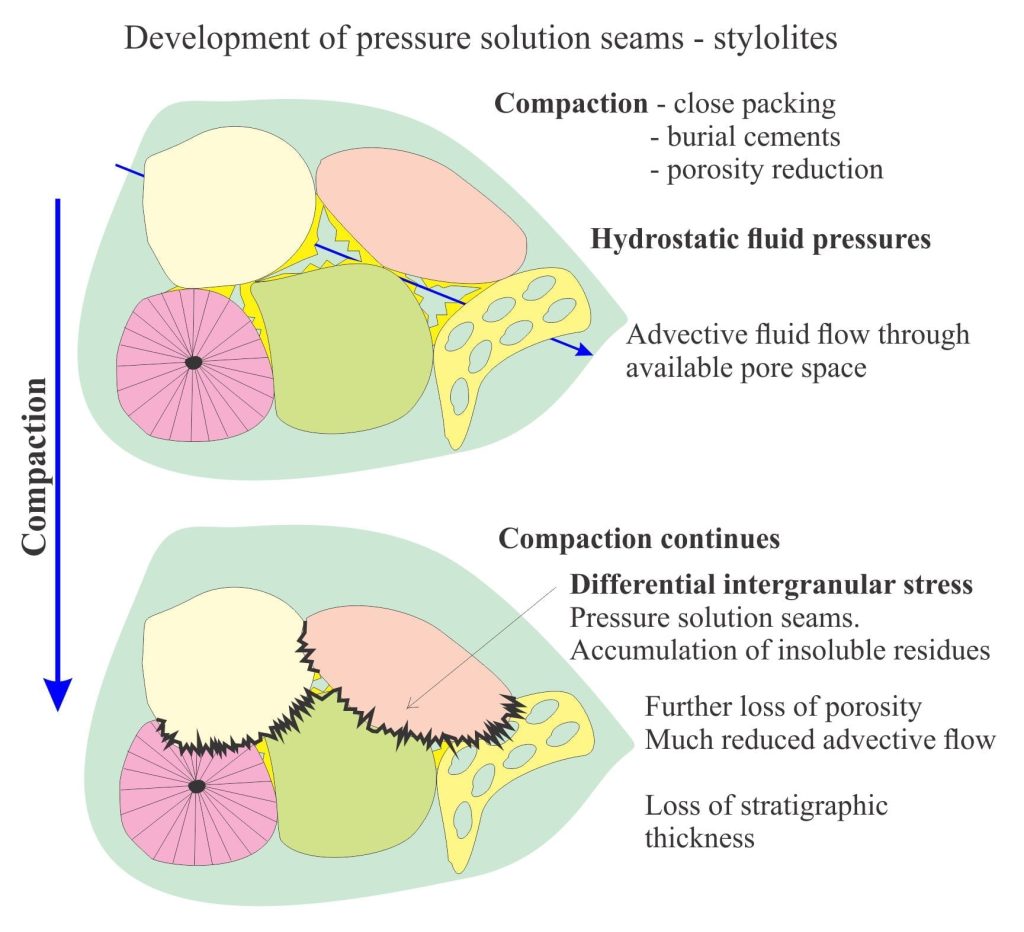
Stress, strain, and fluid flow
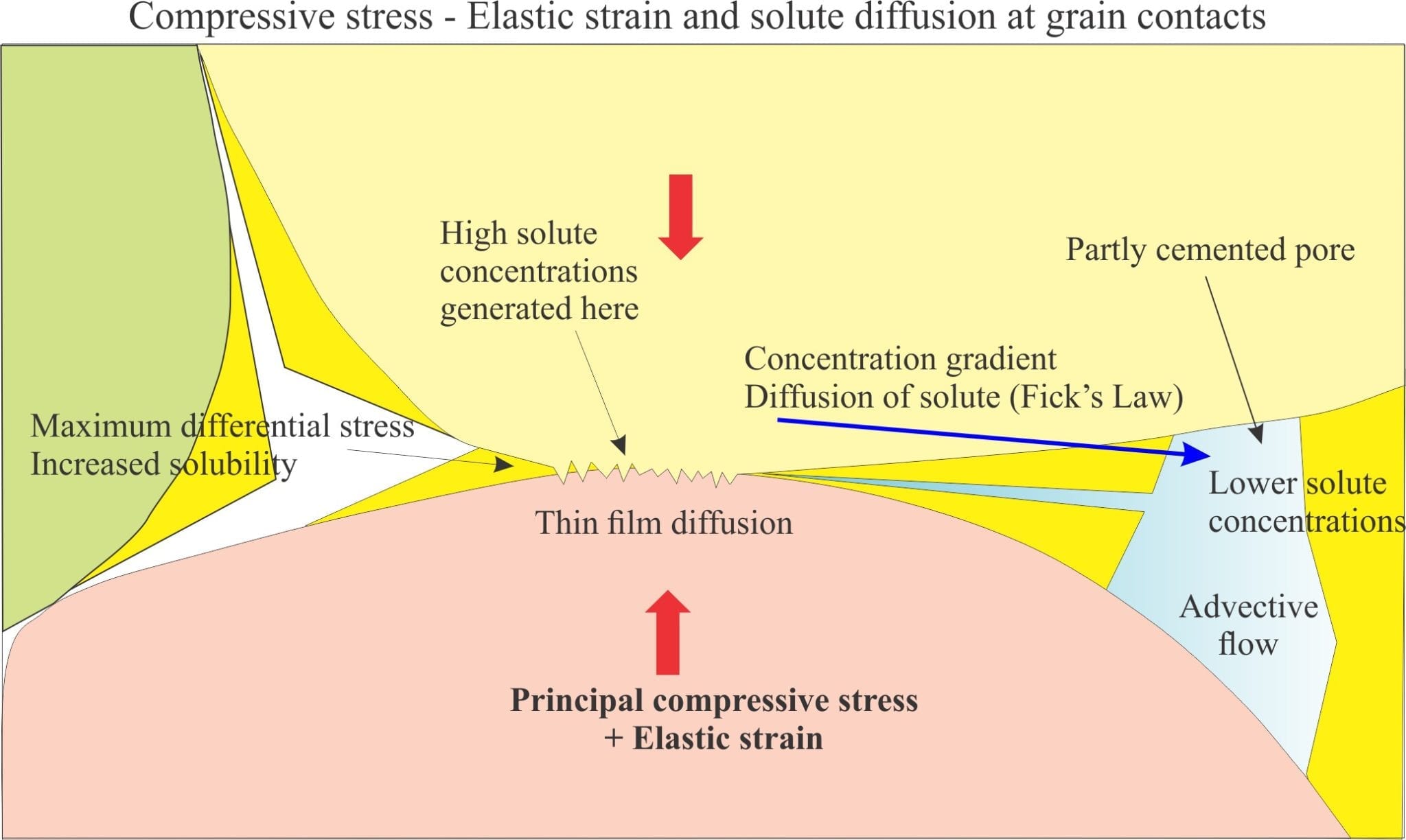
During compaction grains become closer packed, reducing porosity and expelling connate fluids. Sediment rigidity increases, aided by cementation. Fluid pressures at this stage are close to hydrostatic (the pressure due to the overlying column of water and air). At some point in this process differential stresses in excess of hydrostatic develop at grain contacts, raising the potential for calcite dissolution. Dissolution will be a maximum at grain contacts where pressure and strain energy are maximized. The ensuing solute concentration gradient between the contact and adjacent pore space will promote diffusion along a thin fluid film between the grains. Note, if solute cannot be transported from the contact then dissolution will cease. The ‘thin film’ hypothesis was first suggested by P.K. Weyl in 1959. It remains a popular mechanism for explaining solute transport by aqueous ion diffusion.
Diffusion of solute will increase the activity (or concentration) product in the adjacent pore spaces, promoting reprecipitation of calcite (or dolomite); this may take place in the immediately adjacent pore spaces, or solute may be moved farther afield by advective flow (i.e. fluid transport en masse) and precipitate some distance away. Where precipitation occurs will depend on the efficiency of advective flow and the calcite activity product.
Reprecipitation reduces the effective porosity.
The influence of the mechanical properties of grains
One prerequisite for pressure solution to take place is that the grains in contact must be rigid. During compression (compaction) and at the relatively low burial temperatures where pressure solution occurs (less than 200oC) rigid grains will act elastically (unless strain rates are high in which case they behave as brittle materials). Elastic strain energy will contribute, along with compressive stress, to calcite dissolution.
If the materials are ductile then pressure solution is inhibited. Common examples in siliciclastics include lithic grains, and in carbonates non-lithified pellets and mud intraclasts.
As the pressure solution seam increases in length-area, there is a concomitant decrease in effective stress and strain rate. Thus, as seams grow, the tendency for dissolution to decrease acts in concert with a reduction in effective porosity resulting from reprecipitation (i.e. the porosity that permits fluid flow), as a kind of self-regulating process.
The influence of insoluble materials
Stylolites are usually outlined by dark seams of clay, organic matter, and iron oxides – it’s what makes them so recognisable. Clays are particularly important here because they enhance the transfer of dissolved mass away from grain contacts. Most of the insoluble residues were originally dispersed through the host rock and concentrated along the seam as carbonate minerals dissolved.
The role of clays has been debated at length (lots of topics in geology are debated at length). Do clays act as passive bystanders, left behind after all the action, or do they participate in the pressure solution process? A model presented by E. Aharonov and R. Katsman (2009), based on numerical simulation (and not experimental data) posits that clays act as a kind of catalyst to pressure solution. In this model, both clays and compressive stress act together to the extent that stylolites will not propagate if clays are not present.
The consequences of pressure solution
- Pressure solution is the geochemical response to differential stress.
- It is an important part of the diagenesis continuum that produces lithified rock.
- It results in loss of stratigraphic thickness.
- It promotes precipitation that reduces porosity.
Links to other posts in this series:
Mineralogy of carbonates; skeletal grains
Mineralogy of carbonates; non-skeletal grains
Mineralogy of carbonates; lime mud
Mineralogy of carbonates; classification
Mineralogy of carbonates; carbonate factories
Mineralogy of carbonates; basic geochemistry
Mineralogy of carbonates; cements
Mineralogy of carbonates; sea floor diagenesis
Mineralogy of carbonates; Beachrock
Mineralogy of carbonates; deep sea diagenesis
Mineralogy of carbonates; meteoric hydrogeology
Mineralogy of carbonates; Karst
Mineralogy of carbonates; Burial diagenesis
Mineralogy of carbonates; Neomorphism
References etc.
E. Aharonov and R. Katsman, 2009. Interaction between pressure solution and clays in stylolite development: Insights from modelling. American Journal of Science v. 309, p. 607-632.
Robin G.C. Bathurst, 1976. Carbonate Sediments and their Diagenesis. Elsevier, Developments in Sedimentology, 12. 658p. Particularly Chapter 11
R. Tada and R. Siever, 1989. Pressure solution during diagenesis. Annual Review of Earth and Planetary Sciences, v. 17, p. 89-118.
H.C. Sorby, 1863. On the direct correlation of mechanical and chemical forces. Proceedings of the Royal society of London, v.12, p. 583-600. Henry Clifton Sorby was one of the architects of modern sedimentology
P.K. Weyl. 1959. Pressure solution and the force of crystallization – a phenomenological theory. Journal of Geophysical Research, v 64, p. 2001-2025. Weyl promoted the thin fluid film idea in this seminal paper.