This is part of the How To…series on carbonate rocks
Diagenesis of carbonate sediment begins soon after deposition and continues at all depths until metamorphic processes takes over (a statement that is about as diffuse as the boundary between diagenesis and metamorphism). Burial can also be expressed as a function of changing temperature, pressure, and fluid composition, and is manifested as physical and chemical changes such as:
- compaction and pressure solution.
- replacement of metastable and unstable minerals like aragonite, high-Mg calcite, and evaporites by calcite,
- dolomitization,
- creation of secondary porosity, and
- neomorphism – recrystallization.
The term neomorphism was introduced by Folk in 1965. I like Bathurst’s quote of Folk in his own introduction to the topic (Bathurst, 1976 edition, P.477) – neomorphism is a “comprehensive term of ignorance” to embrace “all transformations between one mineral and itself or a polymorph…”. In other words, it includes any mineral recrystallization where the bulk composition does not change, only the size and/or shape of crystals (note that some trace elements and stable isotopes may change). In carbonates, this usually involves neomorphism of aragonite to calcite (a polymorphic change), high-Mg calcite to calcite, or calcite to calcite. Neomorphism also occurs in dolostones.
The term neomorphism does not include void-filling cements; recrystallization only takes place in pre-existing crystals. This stipulation places a fairly serious onus on the petrographer – to distinguish between cement and neomorphic fabrics.
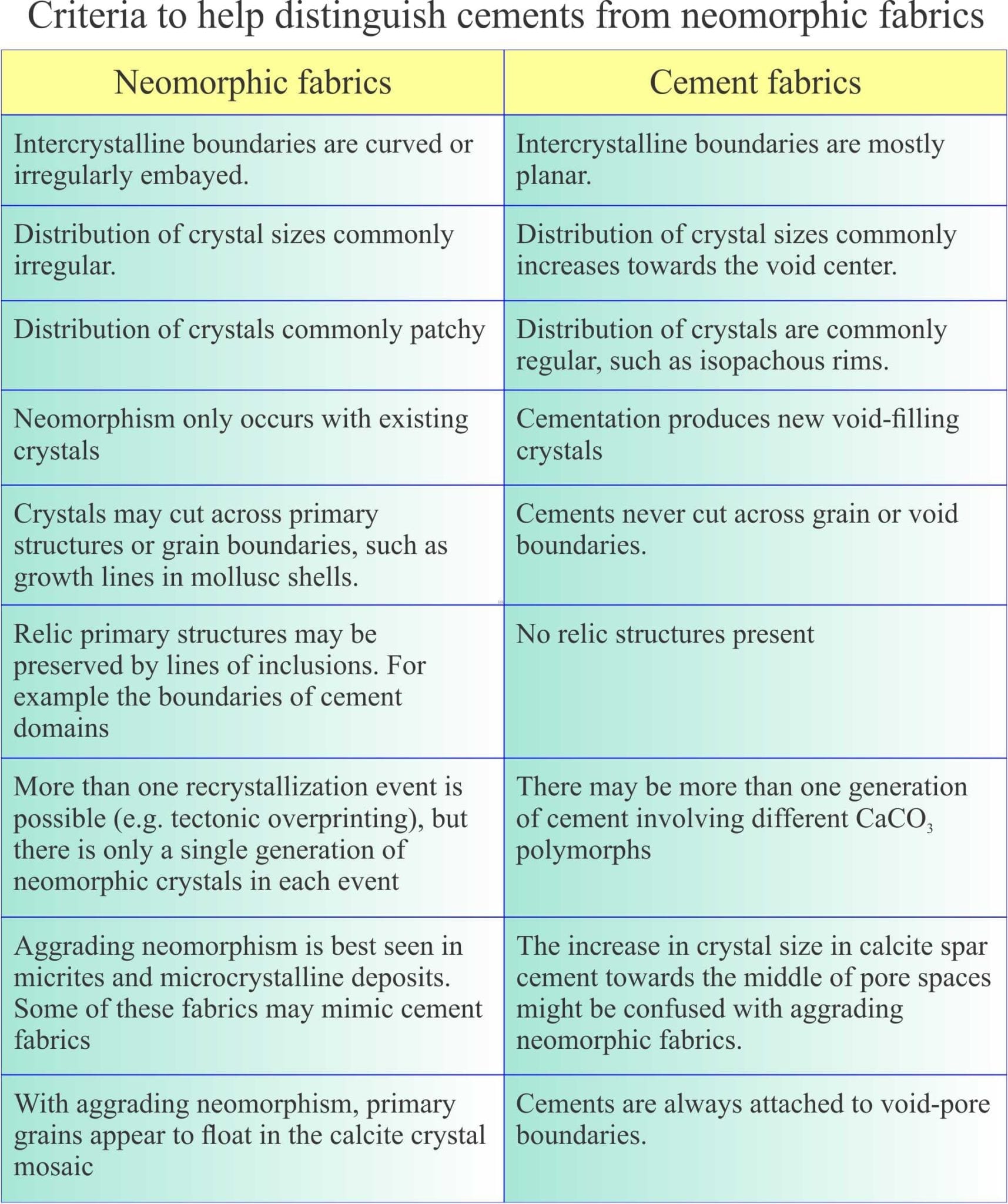
Criteria for recognising neomorphic fabrics
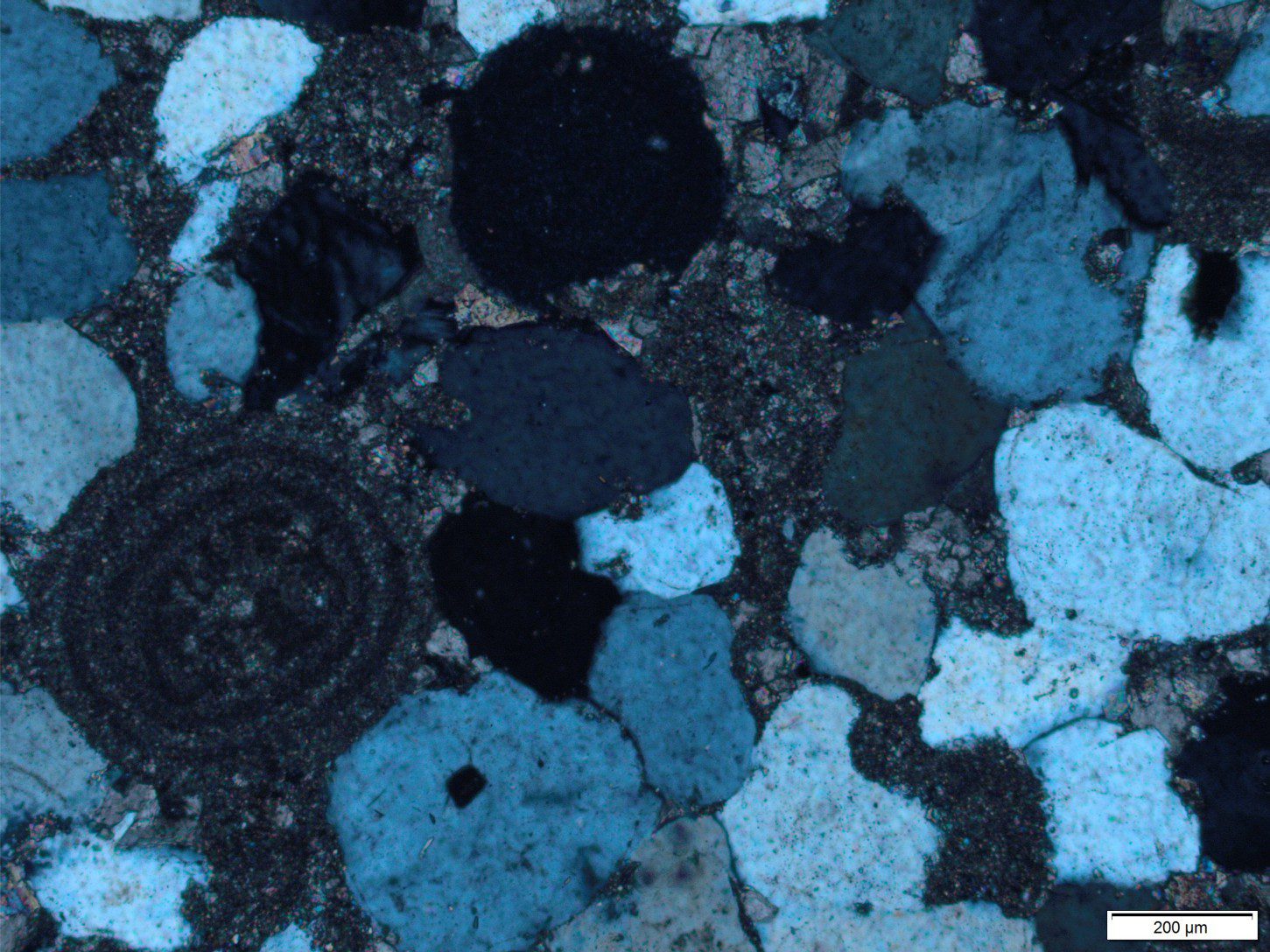
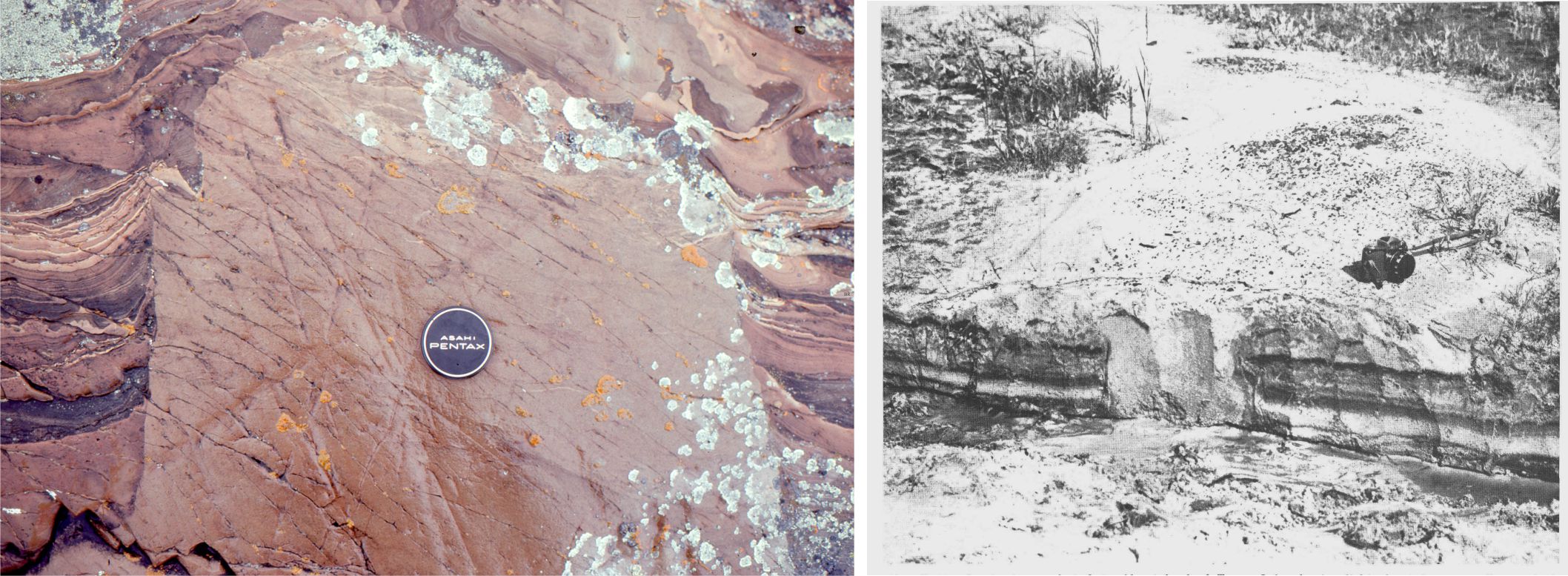
The following criteria help to distinguish between cement and neomorphic textures. Information here has been gleaned from multiple sources, including Folk (1968), Bathurst Chapter 12 (1976), Tucker and Wright (1990), and Flugel, (2010) Chapter 7. Diagnostic criteria include: the geometry of intercrystalline boundaries, the spatial distribution of crystals, relict fabrics such as those that define Cayeux’s structure grumeleuse, and fabrics that cross-cut primary textures and structures as in the example at the top of the page, and the image below.
This image pair below shows contrasting textures and crystal form between cavity filling cements and neomorphism of a (possible) aragonitic mollusc shell. Left is plain polarized light; right crossed polars.

Aggrading neomorphism
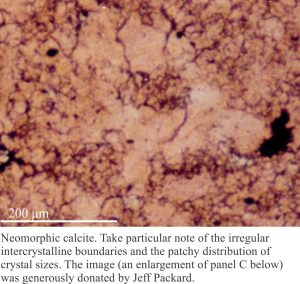
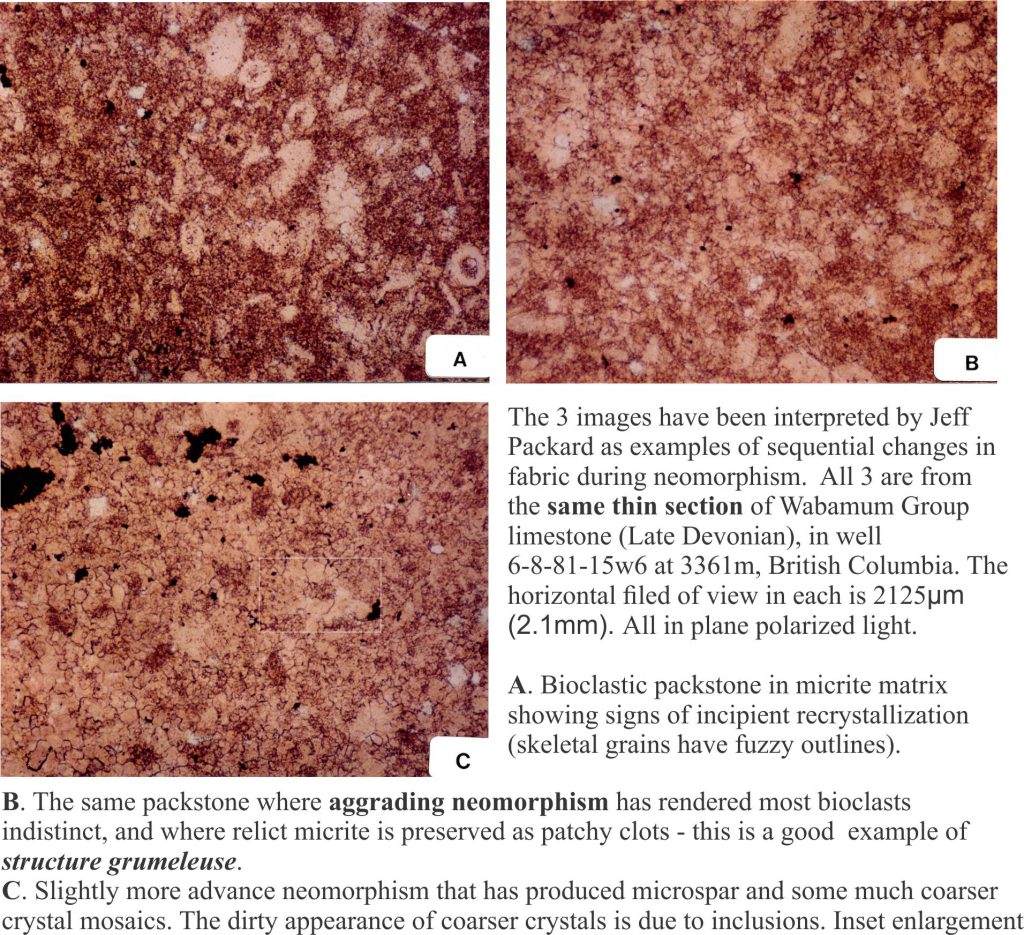
This term is applied to cases where there is a more-or-less regular change in crystal size across a recrystallized domain. The best examples of aggrading neomorphism are found in micrites and lime mudstones, where crystals increase in size in a more-or-less radial fashion, from a relict patch of micrite to coarser neomorphic spar (or pseudospar). Neomorphism may be complete, in which case there is little micrite left, or micrite may remain as diffuse patches or clots that are surrounded by or appear to float in the coarser spar. The clotted fabric is similar to structure grumeleuse, a term introduced by Lucien Cayeux in 1935, and later elaborated by Bathurst (1976).
In some cases, the micrite patches mimic pelloids and have, on occasion, been incorrectly identified as such. Of course, there are many situations where peloidal limestones have been recrystallized. Distinguishing real peloids from neomorphic pseudo-peloids can be a challenge.
Possible Mechanisms
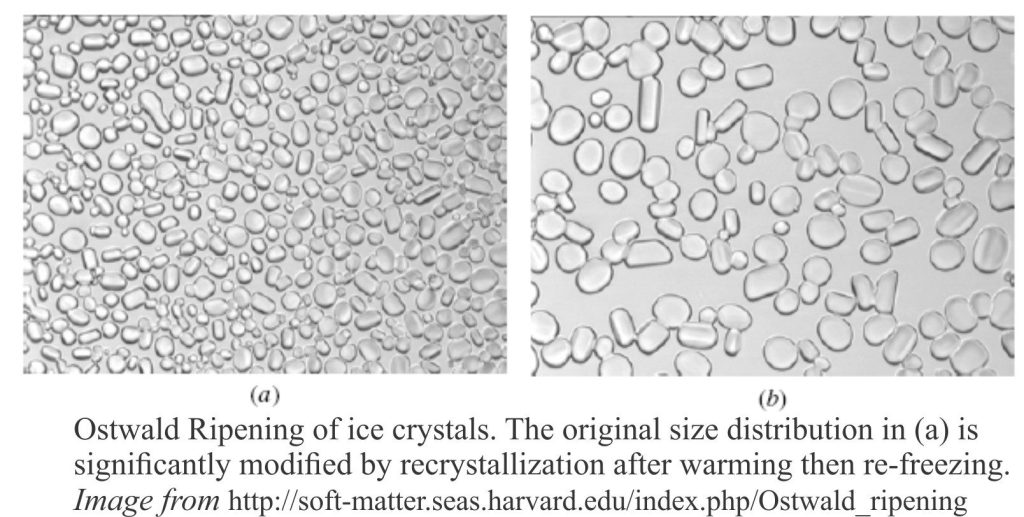
Our general knowledge of recrystallization owes much to the study of crystal annealing in metals, although we can also appeal to substances like ice-cream. In deformed metals, it has been observed that small crystals or grains grow, or recrystallize into larger crystals, a process called Ostwald Ripening (after Wilhelm Ostwald in 1896). The thermodynamic advantage for ripening is the high surface free energy of very small crystals and grains, plus the added free energy caused by defects in the crystal lattices. Metal annealing, or recrystallization takes place in a dry environment.
A more familiar and vaguely annoying example of Ostwald Ripening is found in ice-cream. If ice-cream is warmed slightly then refrozen, the original small ice crystals (that create a smooth creamy texture) recrystallize to larger crystals that give it a course, less pleasant texture. The image below shows Ostwald ripened ice crystals.
In contrast to metals, mineral diagenesis is nearly always water-wet; this is assumed to be true for carbonate recrystallization. However, unlike cementation and pressure solution there is no requirement for mass transfer of solute to or from the site of recrystallization. Recrystallization, it seems occurs at the interface between crystals and thin aqueous films where dissolution and precipitation take place in proximity. Precipitation is probably syntaxial on existing crystals. It is unlikely that dry recrystallization occurs in diagenetic environments.
Details of the mechanism of carbonate neomorphism remain a bit of a mystery. It is possible that, like metals, dissolution is enhanced by crystal lattice defects. Such defects may develop during compaction, changing stress fields during tectonism, or by incorporation of trace metals in the crystal lattice ( for example, Fe, Sr, Mn). Kinetic inhibition of the dissolution-precipitation process may involve these same elements plus certain organic molecules.
The initial size of the carbonate grains or crystals may also play a role. This is illustrated in the classic examples of micrite and microcrystalline calcite neomorphism, like those illustrated above. Sub-micron sized crystals possess greater surface free energy than their coarser counterparts. This, in addition to whatever lattice defects might be present, may help promote dissolution. However, as Morse and McKenzie (1990) point out, the amount of surface free energy decreases exponentially as crystal size increases such that the thermodynamic advantage diminishes rapidly once grain size exceeds about one micron. This limiting condition means that we must search elsewhere for possible mechanisms for neomorphism of coarser fabrics.
Neomorphism is recognised as an important process in the evolution of limestones and dolostones. The search for possible mechanisms of neomorphism in wet diagenetic environments remains fertile ground for debate based on experimentation, field observation and hypothesizing.
Thin section images generously contributed by Vincent Caron and Jeff Packard are indicate on each figure.
Appended
Two recently acquired images comparing cement and neomorphic textures (Feb 14, 2022)
Links to other posts in this series:
Optical mineralogy: Some terminology
Carbonates in thin section: Forams and Sponges
Carbonates in thin section: Bryozoans
Carbonates in thin section: Echinoderms and barnacles
Carbonates in thin section: Molluscan bioclasts
Mineralogy of carbonates; skeletal grains
Mineralogy of carbonates; non-skeletal grains
Mineralogy of carbonates; lime mud
Mineralogy of carbonates; classification
Mineralogy of carbonates; carbonate factories
Mineralogy of carbonates; basic geochemistry
Mineralogy of carbonates; cements
Mineralogy of carbonates; sea floor diagenesis
Mineralogy of carbonates; Beachrock
Mineralogy of carbonates; deep sea diagenesis
Mineralogy of carbonates; meteoric hydrogeology
Mineralogy of carbonates; Karst
Mineralogy of carbonates; Burial diagenesis
Mineralogy of carbonates; Pressure solution
References and other texts
R.L. Folk 1968. Petrology of Sedimentary Rocks
Robin G.C. Bathurst, 1976. Carbonate Sediments and their Diagenesis. Elsevier, Developments in Sedimentology, 12. 658p. Particularly Chapter 12
Erik Flugel. 2010. Microfacies of carbonate rocks: Analysis, interpretation and application. Springer.
J.W. Morse and F.T. Mackenzie 1990. Geochemistry of sedimentary carbonates. Developments in Sedimentology 48. Elsevier, Amsterdam, 707 p. Available as an e-book
H.G Machel, 1997. Recrystallization versus neomorphism, and the concept of ‘significant recrystallization’ in dolomite research. Sedimentology, v.113, p. 161-168. Provides and interesting slant on recrystallization in dolostones