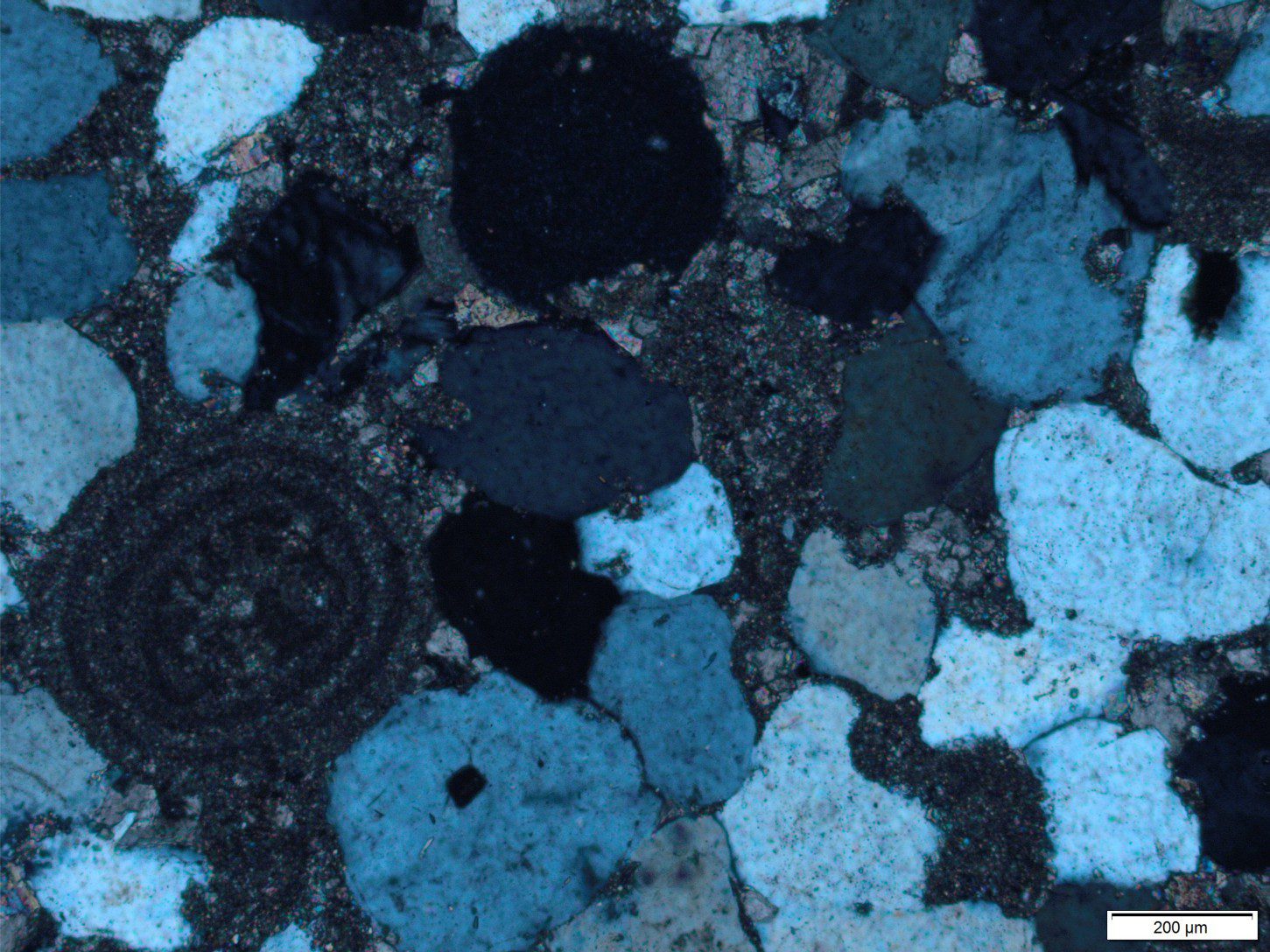
Meteoric diagenesis of carbonates – a competition between dissolution and precipitation.
This is part of the How To…series on carbonate rocks
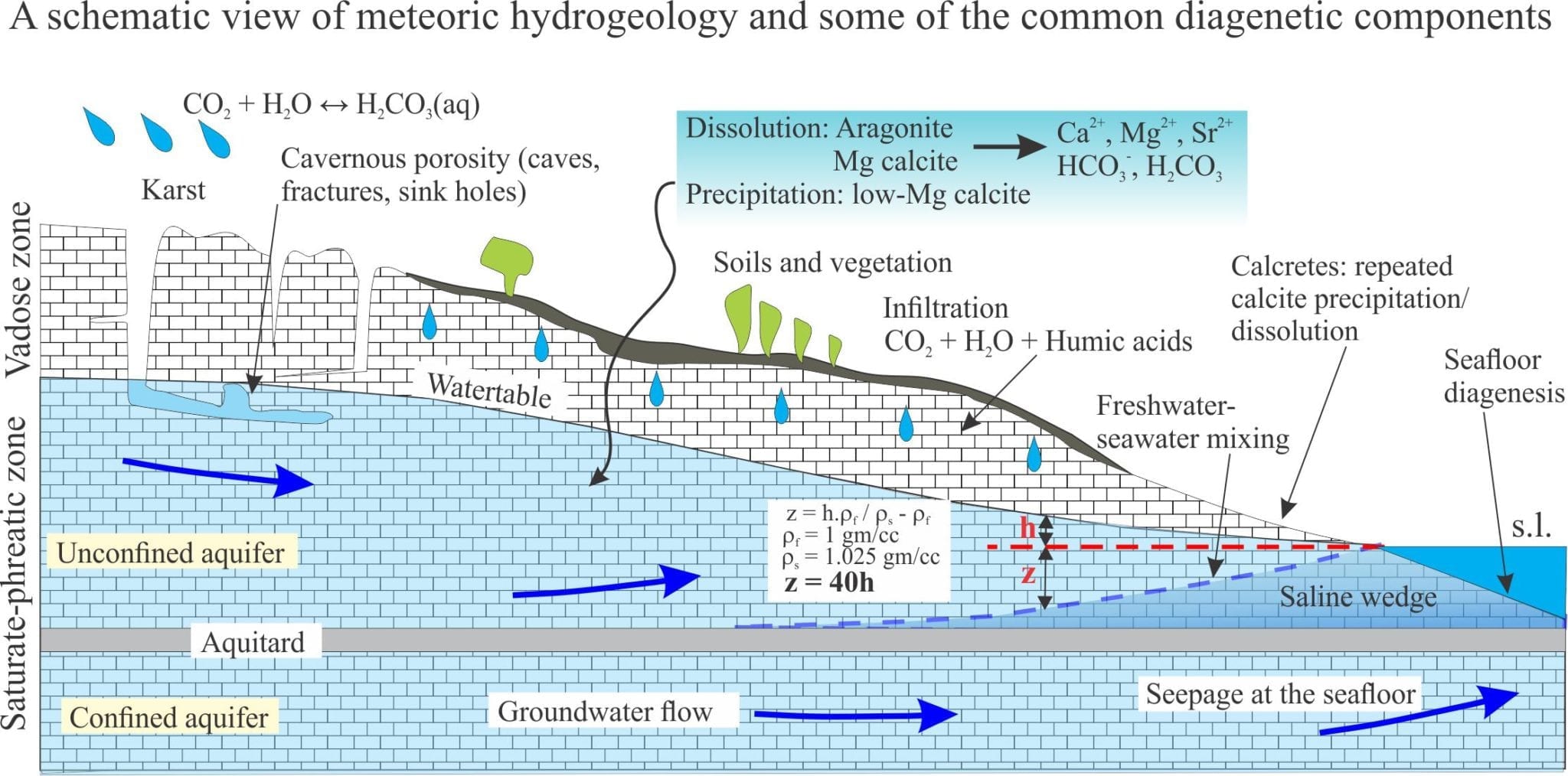
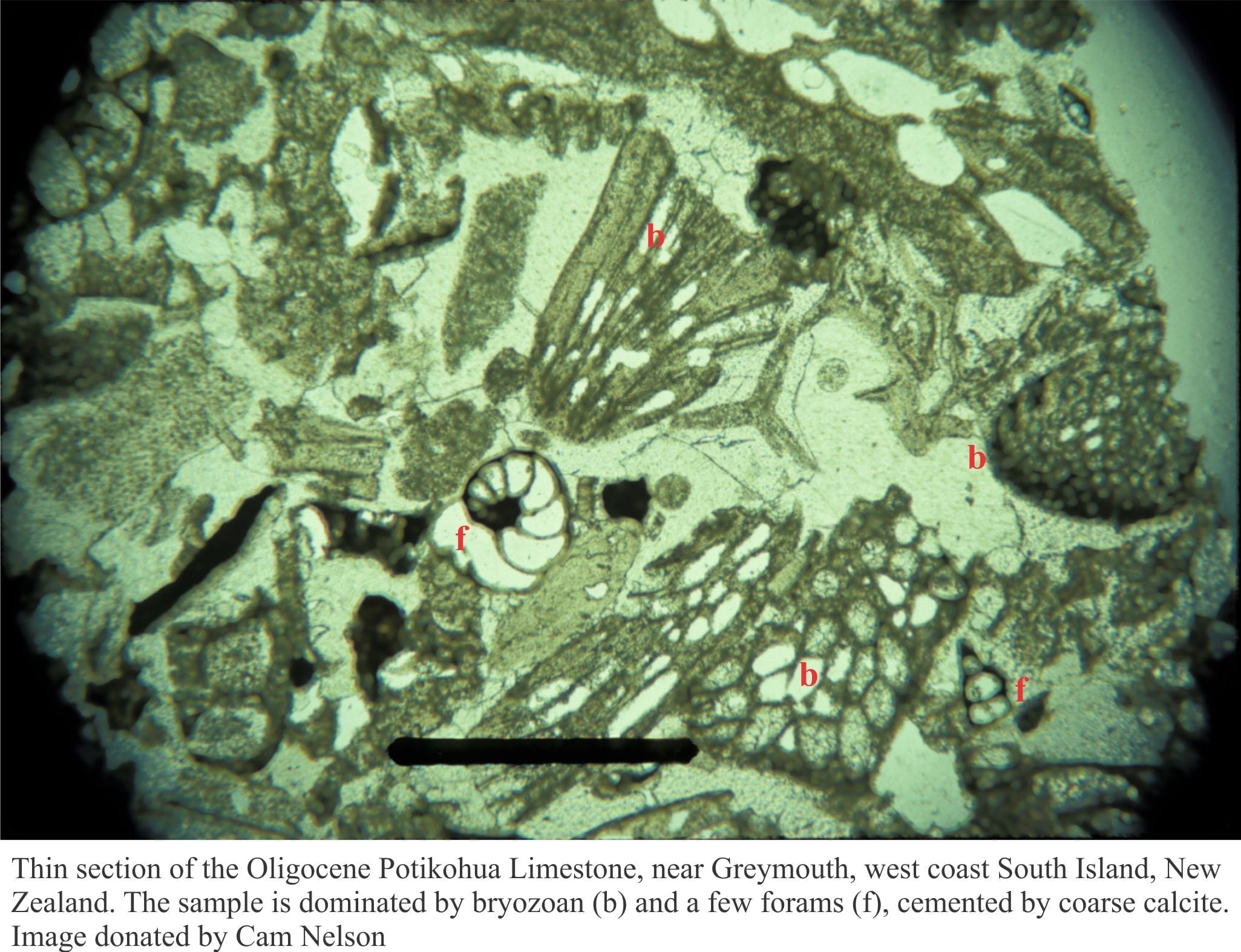
Marine carbonates may, during their geological evolution, be exposed by a fall in relative sea level (eustatic, shoreline retreat forced by progradation, tectonic uplift). The subsequent displacement of seawater by freshwater is rapid, the resulting meteoric diagenesis is profound. The influx of freshwater changes the stability of those mineral phases that were stable or metastable in seawater – principally aragonite, high-Mg calcite in bioclasts, frameworks, ooids and cements. As such, meteoric diagenesis involves significant dissolution of CaCO3. However, experience shows that redistribution of dissolved carbonate results in precipitation in calcretes and caliches, eolianites (cemented dune sands), and deeper pore-filling calcite spar mosaics; most of these cements are low-Mg calcites.
Meteoric diagenesis is entirely dependent on the flow of fresh water to remove dissolved mass (solute) and redistribute it to sites of calcite precipitation. The freshwater itself occurs in two broad settings:
- the surface, where precipitation flows overland as sheet runoff or streams, and infiltrates soils and permeable rock, and
- beneath the surface in aquifers.
Surface diagenesis begins with rain, that is weak carbonic acid because of dissolved CO2 – rain is usually in equilibrium with atmospheric CO2. The average pH of rain is 5.5 to 5.8 ( average seawater pH is 8.0 to 8.1). Corrosion of limestone building materials is an obvious manifestation of calcite dissolution.
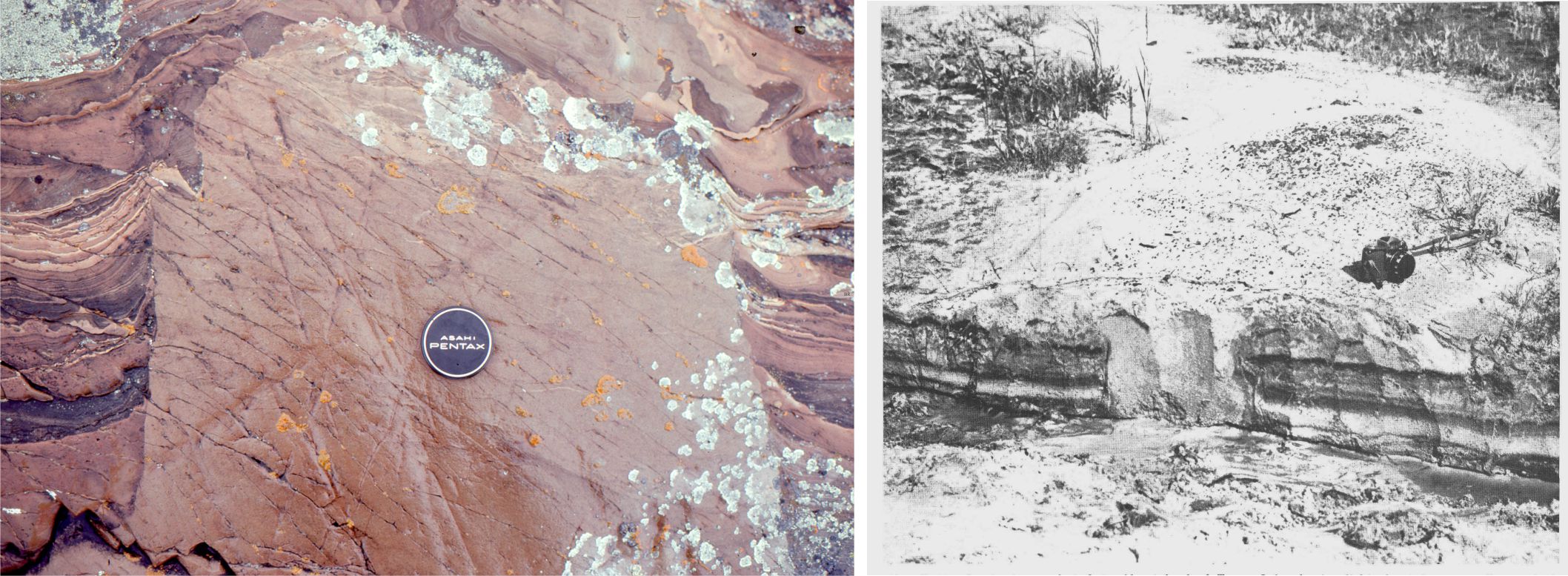
Likewise, development of karst landscapes, sinkholes, caves and subterranean streams are all products of limestone dissolution. Some of the dissolved mass is precipitated as calcretes (caliche, paleosols), fracture-fill spar, stalactites (the ones that hang from the ceiling), stalagmites and cave pearls where waters reach saturation levels and partial pressure of CO2 is reduced.
The unsaturated zone
The interval of infiltration is called the unsaturated zone by hydrogeologists, but limestone aficionados prefer to call it the vadose zone; it extends down to the watertable. It is the interval of porous and permeable sediment or rock in which pore spaces are mostly air-filled and where air pressure is close to atmospheric. Residual water may be present at grain contacts as menisci (because of surface tension); these may act as sites for meniscus cements.
Some of the water in the vadose zone will reach the watertable (groundwater recharge), some of it will be taken up by plants, and some released by evapotranspiration. Two processes act to modify rainwater chemistry:
- Soil formation and break-down of organic matter to humus that releases humic acids and CO2; thus, the partial pressure of CO2 may increase and the pH decrease.
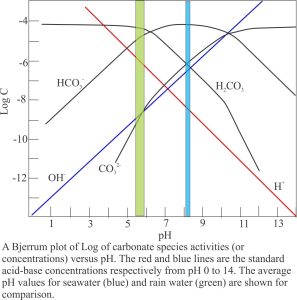
- Dissolution of limestone and carbonate sediment produces Ca2+ and CO32- , but at pH between 5 and 6 the dominant species are HCO3– and H2CO3. A typical plot of species concentration versus pH is shown below.
All these dissolved products enter the groundwater system.
The saturated zone
Pore spaces below the watertable are filled with water; this is the saturated or phreatic zone. Furthermore, groundwater is always on the move, driven for the most part by gravitational potential energy imparted by topography. Porosity is of several types: intergranular, intragranular (e.g. the chambers of gastropods), secondary porosity formed by dissolution of metastable components, fractures, and cavernous porosity formed by solution collapse (sink holes (dolines) and underground streams.
Aquifers
There are two fundamentally different kinds of aquifer:
- Unconfined aquifers, sometimes called watertable aquifers. The watertable is the boundary between the vadose and saturated portions of the aquifer. Drainage in an unconfined aquifer is by gravity. If an unconfined aquifer is drained there is no change in the organization or packing of grains. The position of the watertable fluctuates seasonally, depending on the balance of evaporation, drawdown by vegetation (and people), and recharge.
- Confined aquifers are bound by sediment or rock layers that retard flow (aquitards). They are always saturated (even when drawn down by pumping). In natural systems, there is usually a balance between recharge and discharge of water, but if this balance is upset (e.g. during an extended dry period), then the aquifer framework (i.e. clast support) will respond elastically.
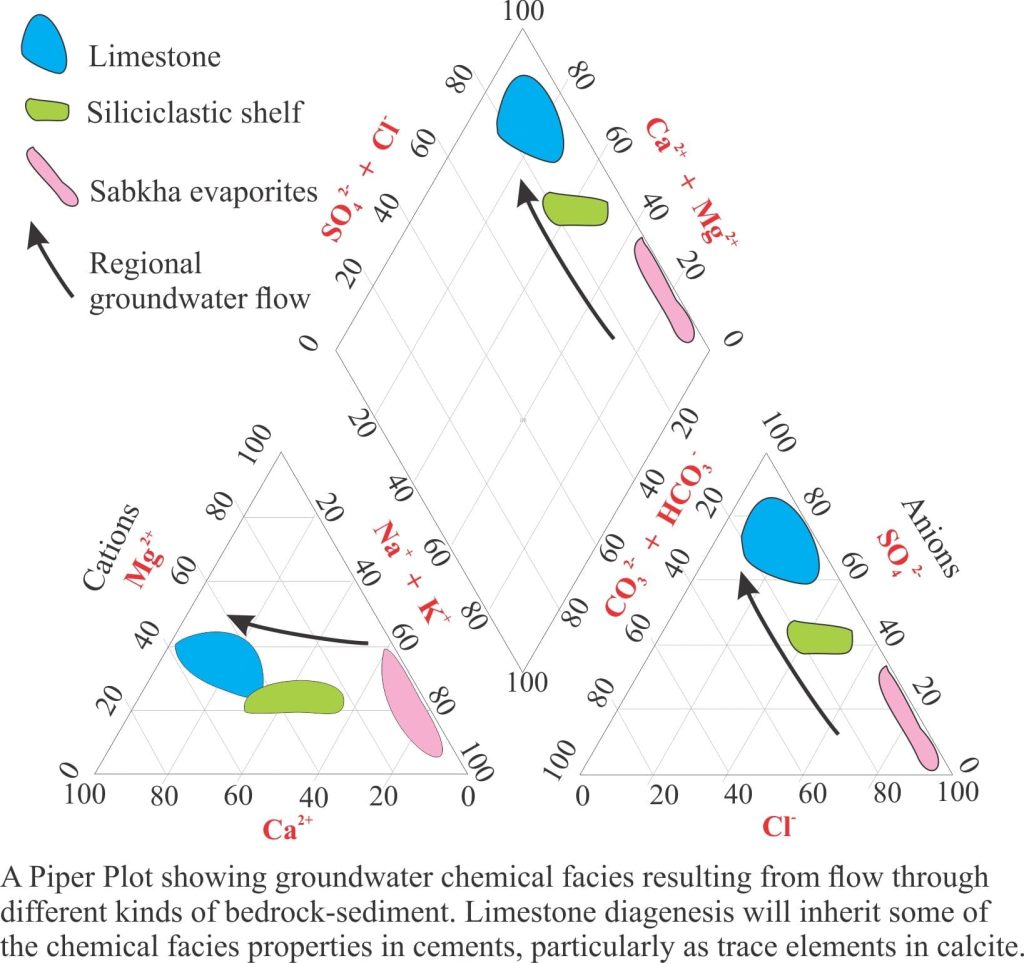
The chemistry of groundwater evolves over time. Rainwater is usually devoid of dissolved salts, but once it enters a groundwater system, biochemical reactions in soils, and inorganic reactions involving carbonates, clays (particularly ion exchange) and other silicate minerals, will add or subtract ion species that can be involved in other reactions. Like their sedimentary cousins, groundwater chemistry can be represented by chemical facies, for example water my be dominated by Ca2+ or Fe3+, or CO32- or SO42-, depending on the composition of aquifer materials. Migration of groundwater through different rock-sediment types is commonly manifested in a change from one chemical facies to another. Tracking this chemical evolution is useful for two reasons: it provides a record of where the water has resided, and may provide clues to changes in the precipitation history of rocks. For example, partitioning of trace elements in zoned calcite cements will record changing pore water compositions.
Groundwater at the shoreline
The interaction between groundwater and seawater can have a profound effect on carbonate diagenesis. Groundwater doesn’t just cease to flow at the shoreline – with enough hydraulic drive it can extend many kilometres beneath the sea floor, exiting as freshwater seepage and springs. One celebrated example is located beneath the Florida carbonate platform where exploratory drilling in 1965 discovered fresh and brackish water in boreholes up to 120km offshore and 130m below the sea floor. In one borehole fresh water flowed 2m above the ship’s deck (Manheim, 1967). Seepage of fresh or brackish water will affect local biotas and the relative stability of metastable carbonates, both in the rocks through which the groundwater flows and at the seafloor. This is illustrated in a great example from Yucatan Peninsula (link kindly provided by Nigel Platt – © Nigel Platt, Edison E&P UK Ltd). Here, fresh-water springs across the shallow platform at Casa Cenote, Tankah Beach upwell through the platform carbonates. Freshwater is recharged from the highlands a few 100 km inland. Flow to the coast is focused through a complex network of limestone caves.
The watertable in coastal unconfined aquifers will tend to merge with the shoreline. Coincidentally, a wedge of seawater will develop landward of the shore below the fresh groundwater. It’s position below the freshwater is dictated by density differences. The boundary between seawater and groundwater is a zone of mixing. A reasonable approximation of the depth to the groundwater-seawater boundary is expressed in the Ghyben-Herzberg equation, shown in the diagram above:
z = h.ρf / ρs – ρf
where z is the depth to the interface from sea level, h the watertable elevation, ρs (1.025 gm/cc) and ρf (1 gm/cc) the densities of seawater and freshwater respectively, such that
z = 40h
An important corollary is that for every unit decrease in watertable depth (h) there will be a corresponding 40 unit rise in the interface (and vice versa).
The interface is dynamic and will respond to both short term changes (e.g. seasonal fluctuations in recharge, pumping) and longer geological intervals, migrating with excursions of the shoreline during rising or falling sea levels. Again, from a geological perspective, this means that boundary between fluid environments that determine carbonate mineral stability, will also be dynamic.
Links to other posts in this series:
Mineralogy of carbonates; skeletal grains
Mineralogy of carbonates; non-skeletal grains
Mineralogy of carbonates; lime mud
Mineralogy of carbonates; classification
Mineralogy of carbonates; carbonate factories
Mineralogy of carbonates; basic geochemistry
Mineralogy of carbonates; cements
Mineralogy of carbonates; sea floor diagenesis
Mineralogy of carbonates; Beachrock
Mineralogy of carbonates; deep sea diagenesis
Mineralogy of carbonates; Karst
Mineralogy of carbonates; Burial diagenesis
Mineralogy of carbonates; Neomorphism
Mineralogy of carbonates; Pressure solution
Mineralogy of carbonates; Sabkhas
References and useful texts
Manheim, F.T. 1967, Evidence for submarine discharge of water on the Atlantic continental slope of the southern United States, and suggestions for further research New York Academy of Sciences Transactions, Series 2, v.29, p. 839-853.
Robin G.C. Bathurst, 1976. Carbonate Sediments and their Diagenesis. Elsevier, Developments in Sedimentology, 12. 658p. An example of the longevity and utility of one of the best on this topic. Now also as an ebook.
Noel James and Phillip Choquette.1984. Diagenesis 9. Limestones; The meteoric environment. The Canada Geoscience Series on Carbonate Diagenesis is available from the CGS archive.
Noel James and Brian Jones. 2015. The origin of carbonate sedimentary rocks. American Geophysical Union, Wiley works, 464p.An excellent recent update.
Peter Scholle and Dana Ulmer-Scholle, 2003. A colour guide to the petrography of carbonate rocks: grains, textures, porosity, diagenesis. AAPG Memoir 77. Loaded with images.
Erik Flugel. 2010. Microfacies of carbonate rocks: Analysis, interpretation and application. Springer. The ebook is cheaper