Carbonate sediments and diagenesis in the deep oceans. This is part of the How To…series on carbonate rocks
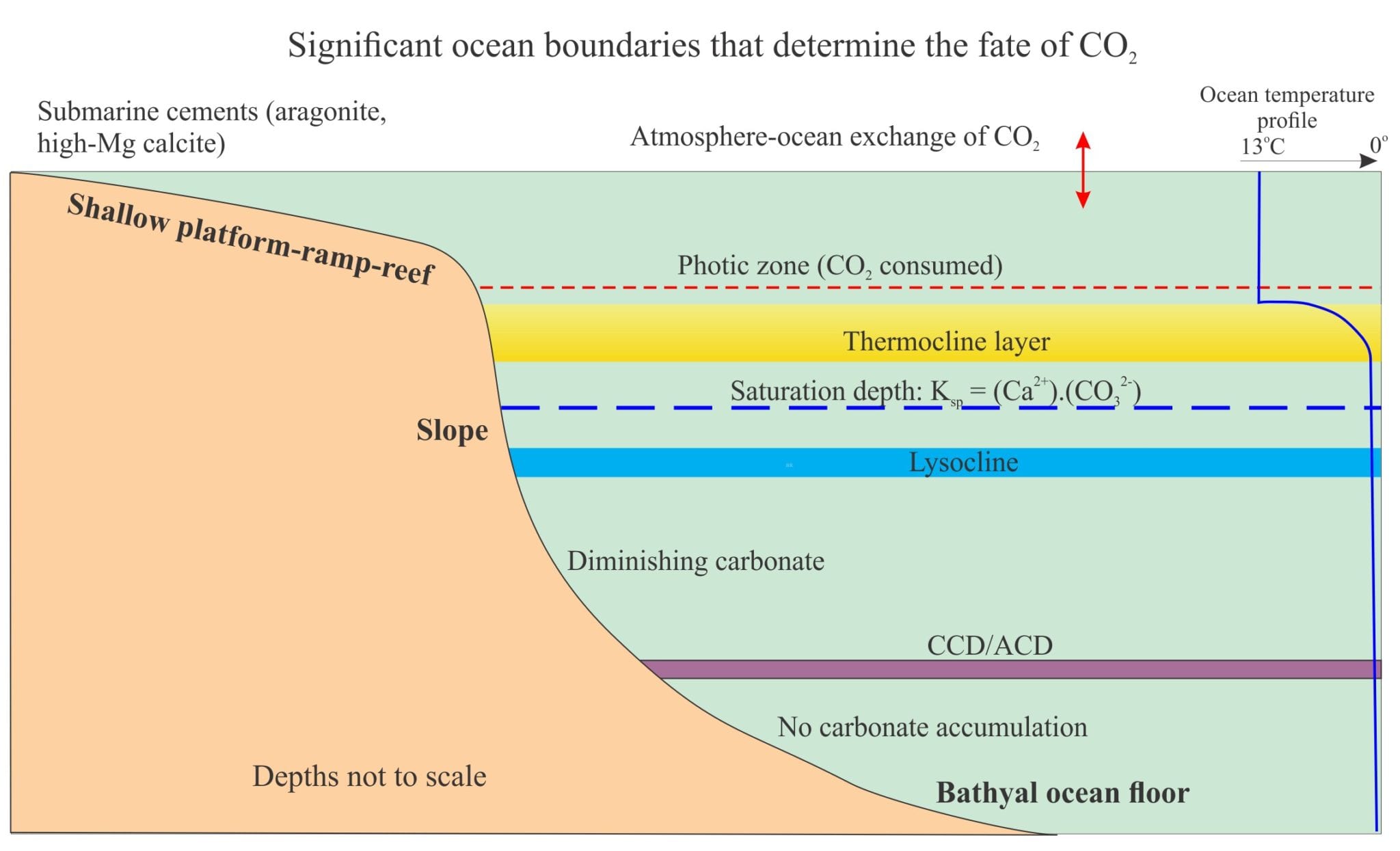
Seafloor diagenesis on shallow carbonate platforms, ramps, reefs and lagoons is mainly a product of precipitation – aragonite and high-Mg calcite in tropical-subtropical seas, low-Mg calcite in cooler waters. Diagenesis continues farther offshore on slopes and the deeper ocean floors, but the biological, physical and chemical conditions change. As water depth increases, we are confronted by several boundary conditions that dictate the fate of CO2 and the carbonic acid system – here the primary reactions involve the dissolution of CaCO3.
Two boundaries are important in shallow seas:
- the photic zone where CO2 is consumed during photosynthesis (to about 200m depth), and
- the thermocline, the layer extending from about 200m to 1000m depth where the temperature decreases rapidly. Below the thermocline the water temperature varies little from about 4o Above the thermocline, CO2 is derived mainly from exchange with the atmosphere. Seawater here is everywhere supersaturated with respect to calcite and aragonite by factors of 4 to 6 times in tropical seas, less in polar waters.
In waters below the thermocline, CO2 fluxes are a product of oceanic circulation and, in contrast to shallow water conditions, oxidative degradation of organic matter that produces CO2. As depths increase, the partial pressure of CO2 increases and the temperature decreases – in both cases CaCO3 becomes increasingly soluble. An important consequence of this convergence is a decrease in CaCO3 saturation to the point where calcite and aragonite begin to dissolve. The important equilibria here is (but note the other equilibria involving CO2 will also be involved):
CaCO3 + CO2 + H2O ↔ Ca2+ + HCO3–
Outcomes of research into water depth-stability relationships for calcite (and aragonite) have identified three additional boundaries:
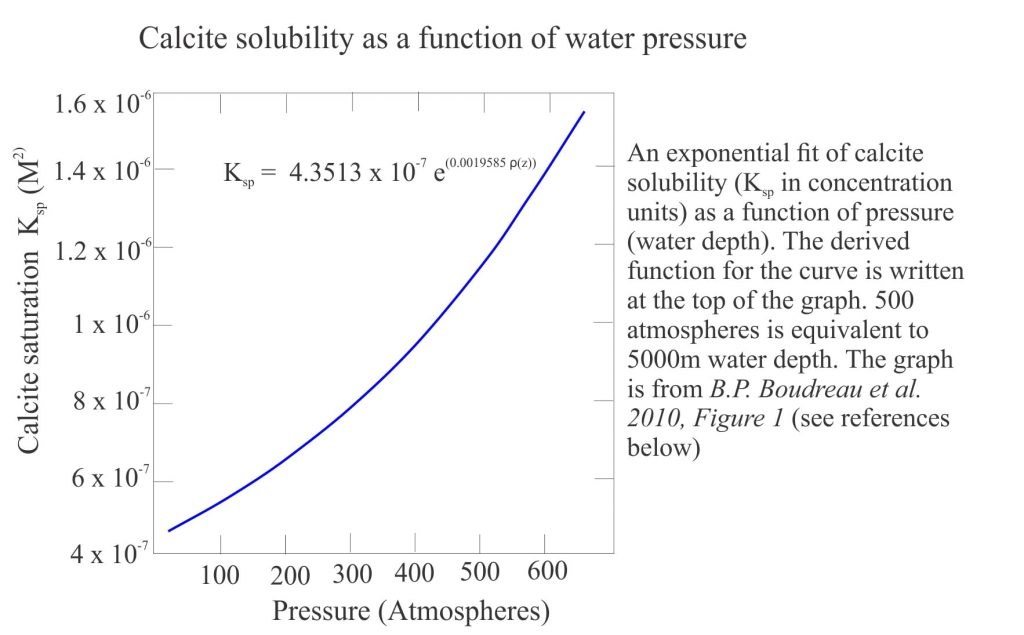
- The Saturation depth that identifies when seawater becomes unsaturated with respect to calcite (or aragonite). The saturation depth is determined by comparing the measured solubility product of either the activity or concentrations of Ca2+ and CO32- with the equilibrium solubility product. The generalized plot below shows calcite solubility increasing with pressure.
- The Lysocline, that is placed at a depth where the dissolution of calcite is first observed in sediment. It was first defined by Berger (1971) who experimented with calcite foraminifera samples suspended on a line at different depths in central Pacific Ocean (for about 4 months). This enabled him to define the Lysocline in equatorial Pacific at about 4000m. Theoretically the Lysocline and Saturation depth should be close. However, the Lysocline is described as subjective because of the practical difficulty recognizing initial dissolution in sediment particles.
- The Calcite Compensation Depth (CCD), which is the depth on the ocean floor where the rate of calcite dissolution (of foraminifera, coccoliths, and aragonitic pteropods) equals the rate of supply from the water column. Thus, there is no net increase in carbonate content. Note that the CCD is a characteristic of seafloor sediment. As such, some have defined it in more practical, geological terms as the depth where the carbonate content of sediment is <20% (Morse and Mackenzie 2010; Broecker, 2008 – see references below).
There is some variation in CCD depths among the oceans. Current depths in the Indian and Pacific oceans are about 4.6km, the North Atlantic 5.1km. Apparently the difference is due to higher CO32- concentrations in deep North Atlantic waters. For aragonite, the ACD is significantly shallower because of its higher solubility compared with calcite; typically it is about 3000m above the CCD.
Slope rhythmites
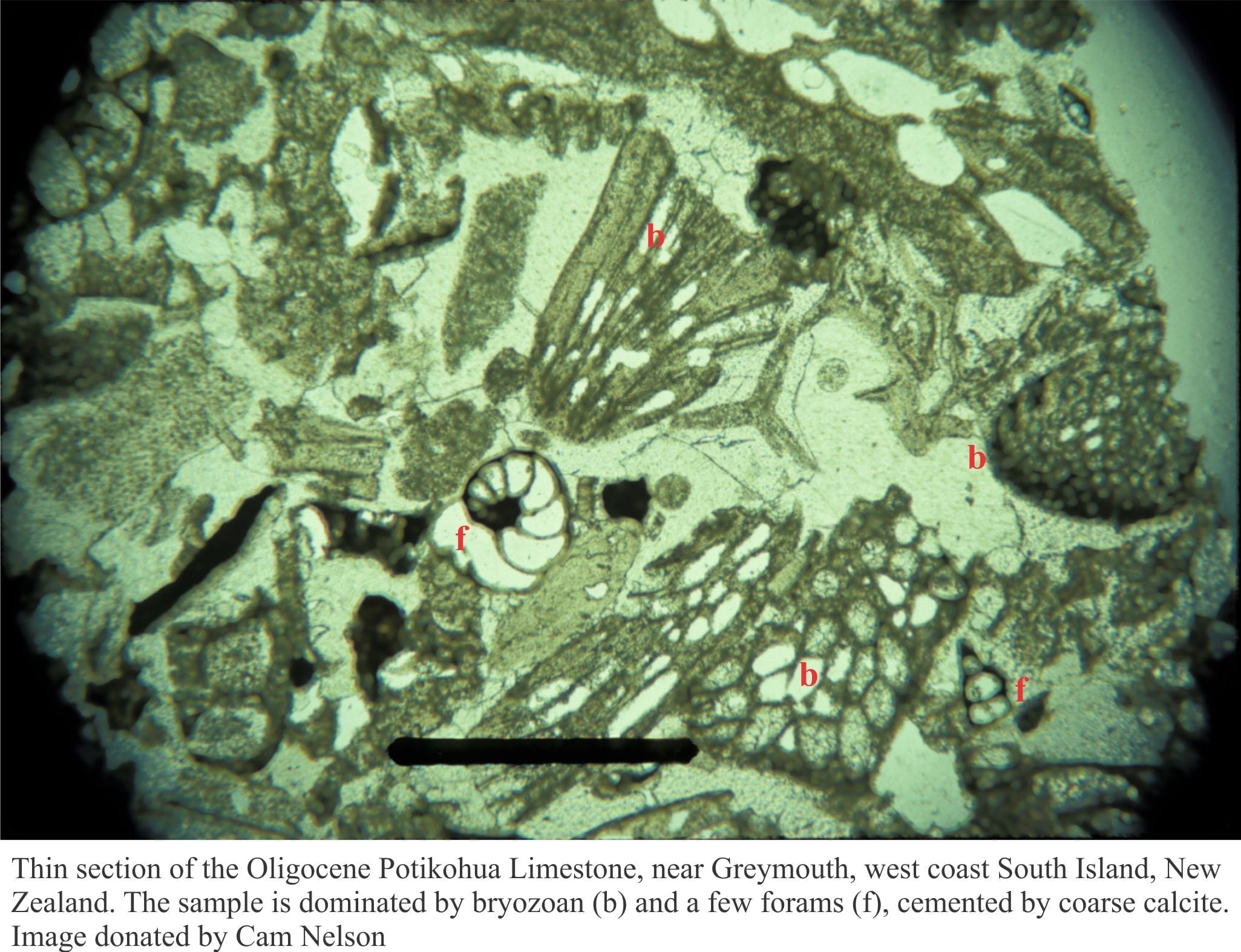
Although CaCO3 becomes less stable below the Saturation depth, its precipitation at shallower levels contributes to hardground formation on ramps and slopes adjacent to carbonate platforms. Modern pelagic carbonates consist of foraminifera, coccoliths, and aragonitic pteropods that have settled through several 100m to the seafloor. This kind of pelagic ooze is characteristic of the Mesozoic and Cenozoic, but less so or absent from the Paleozoic and Precambrian. Classic examples include the coccolith-dominated Cretaceous chalks of Europe.
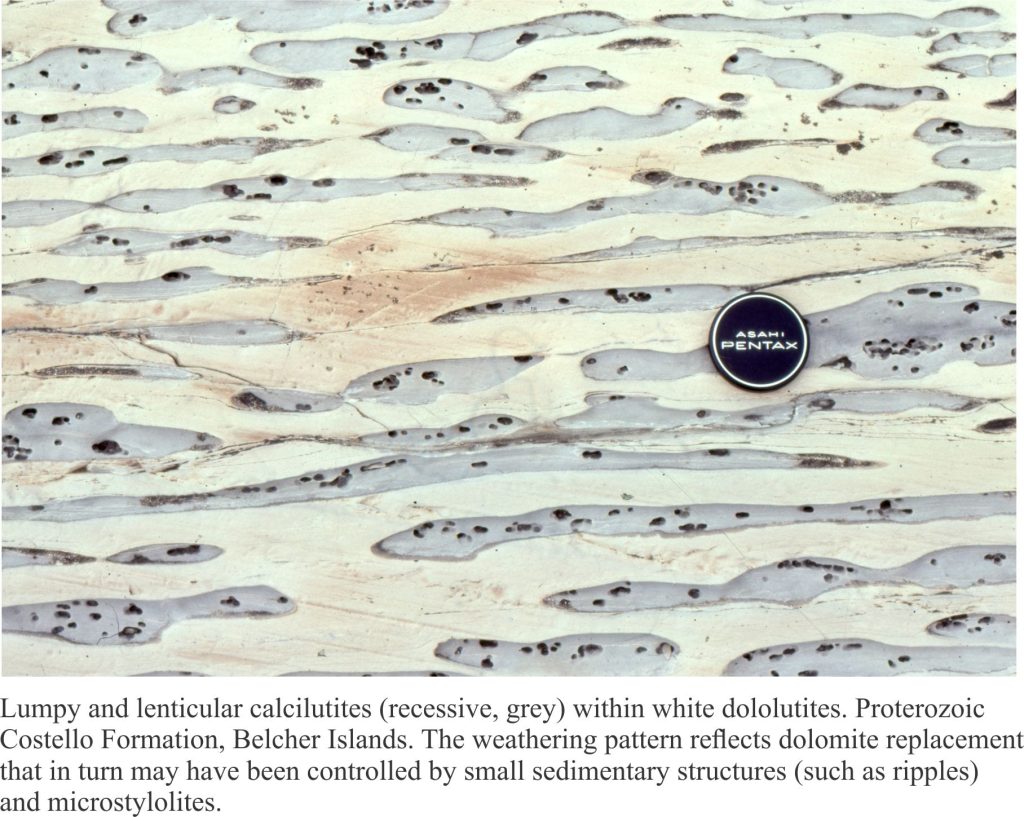
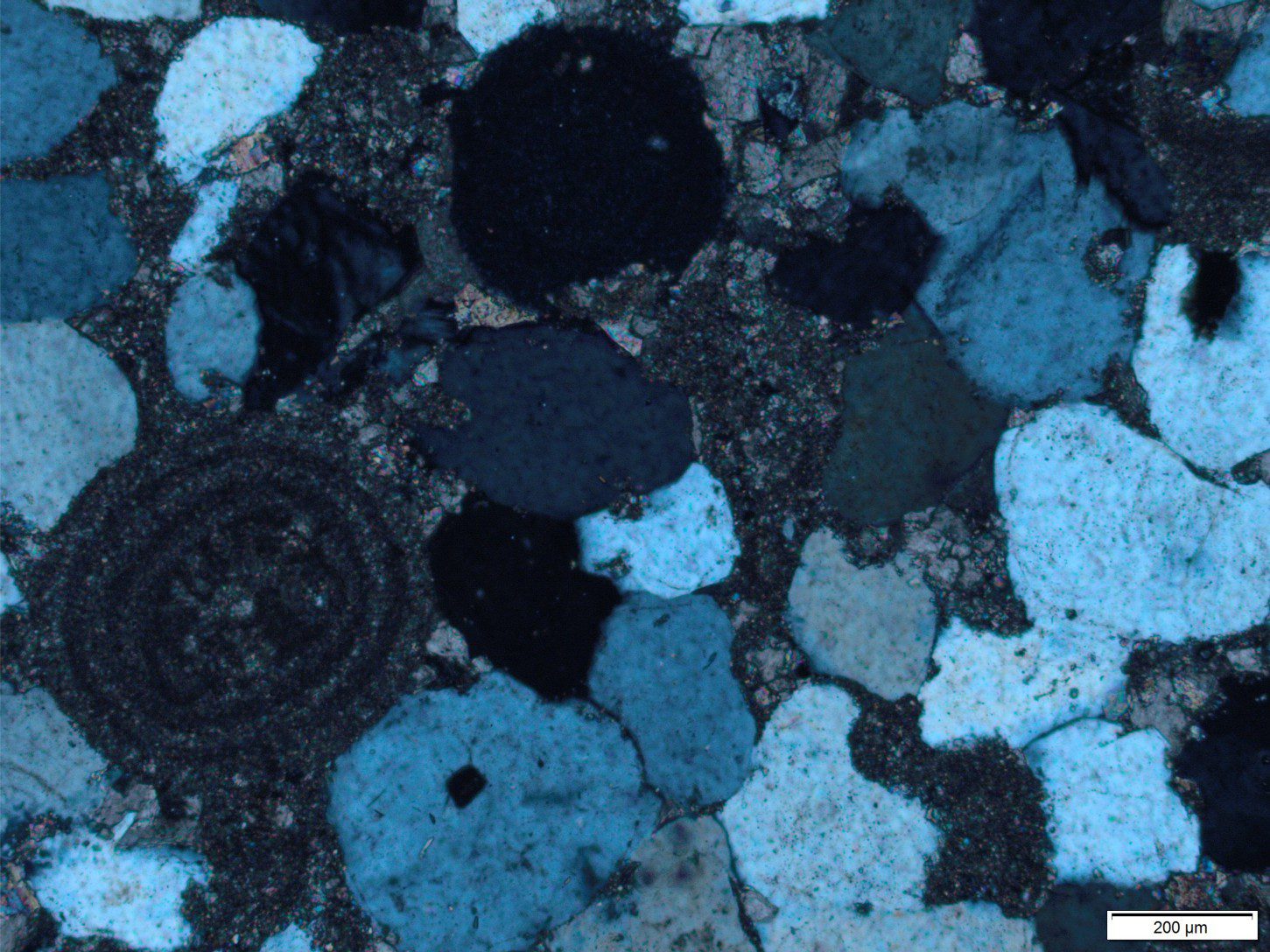
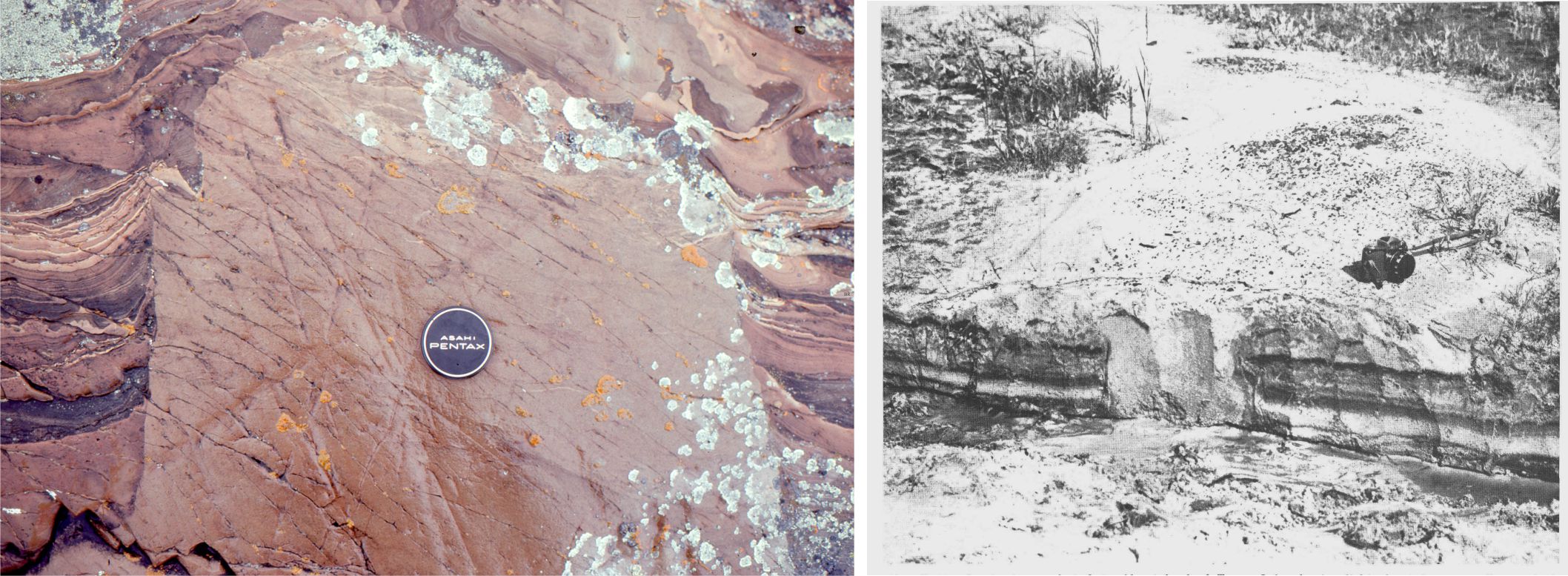
Slope carbonates (rhythmites) from the Proterozoic Belcher Group provide illustrate some of the characteristic features of hemipelagic (mixed carbonate, terrigenous clay and silt) and limestone-dolostone deposits. This is the Costello Formation, characterised by thin, rhythmically bedded calcilutites and dololutites, interbedded with more clay-silt rich layers. Costello slope carbonates were located basinward of a carbonate platform containing extensive stromatolite buildups. Much of the carbonate mud and terrigenous clay-silt deposited on the slope was derived from adjacent platform.
The red colours are due mainly to clays and iron oxides. Small current ripples in some coarser-grained beds indicate modest bottom currents. Graded beds, some containing Bouma cycles indicate resedimentation by turbidites. Small slump packages involving a few metres of stratigraphy and sandwiched between undeformed strata, developed via slope instability.
Flat pebble conglomerates occur as shallow channel fills as well as laterally continuous beds. The flat pebbles were locally derived; there are also some fragments of cryptalgal laminates that probably were derived from the platform.
The flat pebbles, up to 30cm long, do not show any indication of soft-sediment deformation; they may represent disrupted carbonate hardgrounds on the ancient slope. Later diagenetic features include dolomite replacement of CaCO3. The lumpy, lens-like appearance of the beds is partly due to dolomite replacement (white-yellow) plus pressure solution (stylolites).
Links to other posts in this series:
Mineralogy of carbonates; skeletal grains
Mineralogy of carbonates; non-skeletal grains
Mineralogy of carbonates; lime mud
Mineralogy of carbonates; classification
Mineralogy of carbonates; carbonate factories
Mineralogy of carbonates; basic geochemistry
Mineralogy of carbonates; cements
Mineralogy of carbonates; diagenetic settings
Mineralogy of carbonates; seafloor diagenesis
Mineralogy of carbonates; Beachrock
Mineralogy of carbonates; meteoric hydrogeology
Mineralogy of carbonates: Stromatolite reefs
References
W.H.Berger. 1970. Planktonic foraminifera: selective solution and the Lysocline. Marine Geology, v. 8, p.111-138.
B.P.Boudreau, J.J. Middleburg, and F.J.R. Meysman, 2010. Carbonate compensation dynamics. Geophysical Research Letters, v. 37, L03603.
W.S. Broecker. 2008. A need to improve reconstructions of the fluctuations in the calcite compensation depth over the course of the Cenozoic. Paleooceanography, v. 23, PA1204.
J.W. Morse and F.T. Mackenzie 1990. Geochemistry of sedimentary carbonates. Developments in Sedimentology 48. Elsevier, Amsterdam, 707 p.