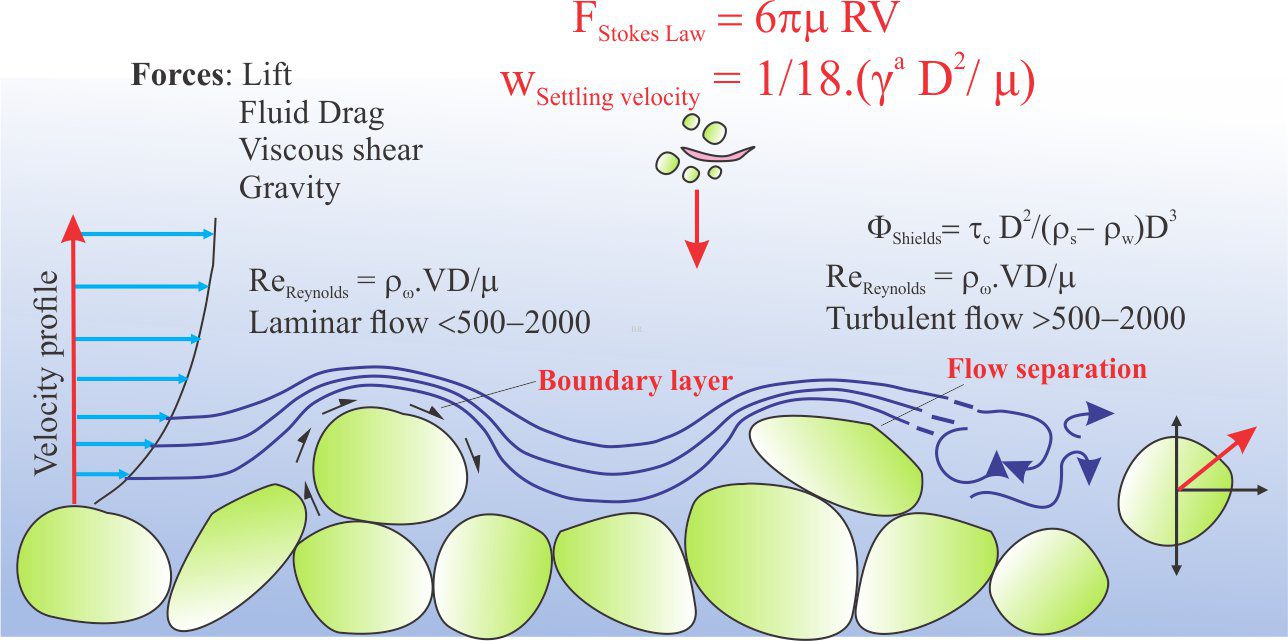
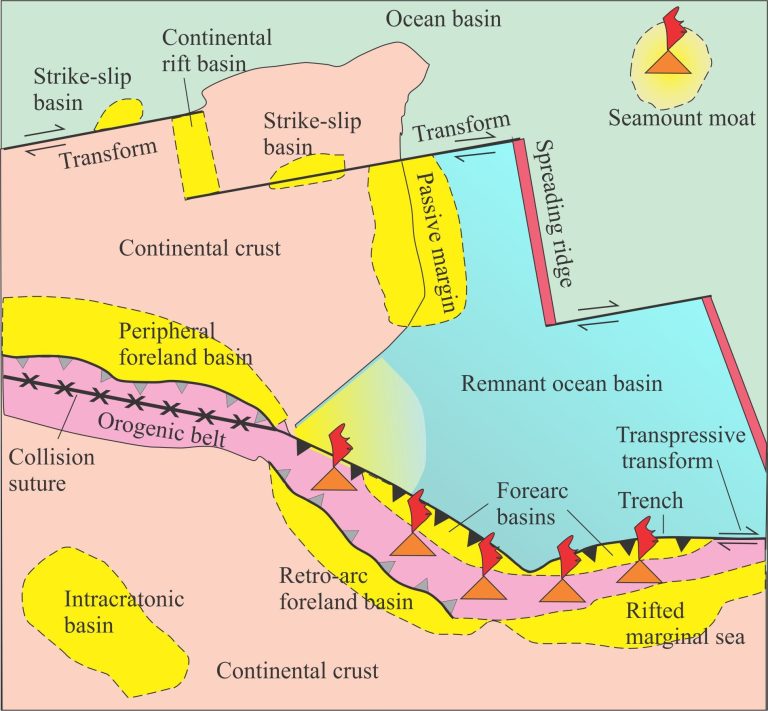
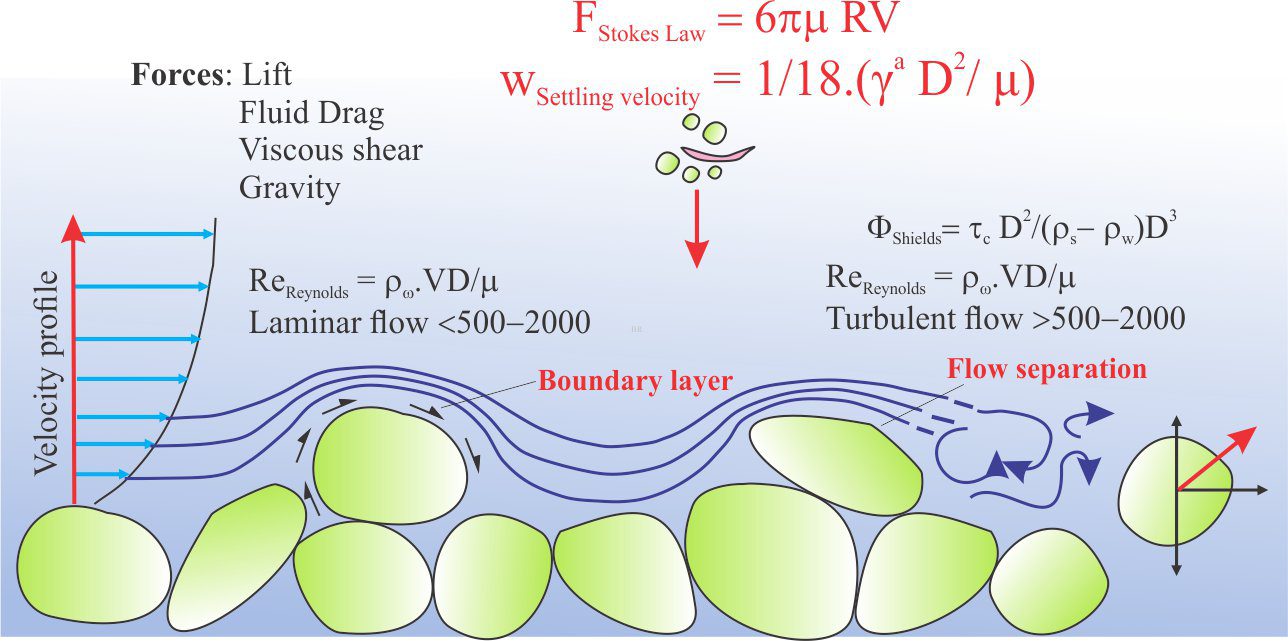
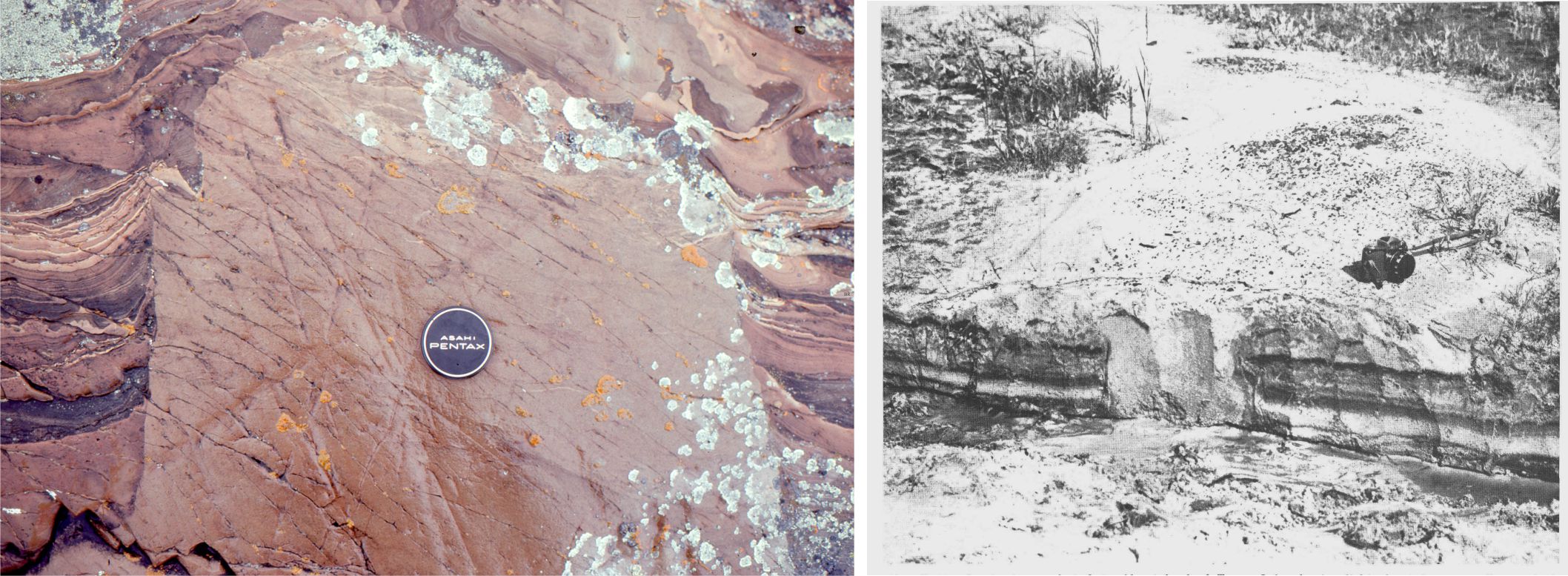
Carbonate sediment is produced in carbonate factories.
This is part of the of How To…series… on carbonate rocks
Most carbonate sediments are precipitated locally then redistributed across the sediment-water interface and through the water column; a kind of in-house production. Carbonate sediment is produced by biological and abiotic processes under conditions determined primarily by light intensity (the photic zone), temperature, salinity, nutrients, biologically available oxygen and water chemistry. The concept of carbonate factories was introduced in the late 1990s to focus attention on regions of benthic carbonate production where these environmental determinants reach a kind of nexus (some important contributions are listed at the end of this article).
The contribution to carbonate production by biotic and abiotic processes is shown in the diagram below. Abiotic production involves direct precipitation of calcite and aragonite and if truly abiotic, depends only on thermodynamic conditions such as temperature, ion activity and the degree of saturation with respect to each mineral, and free energy. It includes sea floor cements, whitings and ooids, although it is becoming increasingly apparent that the last two also involve biotic processes.
The involvement of biotic processes in carbonate production includes:
- Biological activity that influences or mediates carbonate precipitation, but the minerals are not part of the organism’s structure. Thus, the metabolism of some bacteria, cyanobacteria and red and green algae can modify the local chemistry (e.g. alkalinity, pH, ion activity) and promote precipitation.
- Organisms that produce calcium carbonate in shells, skeletons and rigid frameworks that are fundamental to their existence. In Tropical Factories, this includes a very large group of molluscs and other invertebrates like corals, bryozoans. All these critters are heterotrophs that derive their energy by consuming other organisms. The other important group are the autotrophs that manage their energy pathways using external sources like sunlight (i.e. photosynthesis) by creating molecules like carbohydrates that store energy. The autotrophic group includes bacteria, photosynthetic algae such as Halimeda and Penicillus and robust calcareous algae like Lithothamnion which, as we have seen, are important produces of lime mud.
The production lines
The rationale for introducing the concept of carbonate factories is based on the recognition of geologically and geographically recurring facies and associated biotic and abiotic production systems. An obvious advantage of the concept is to focus attention on the dominant environmental and biochemical controls on carbonate precipitation in ancient systems. One disadvantage is that subordinate processes might be sidelined.
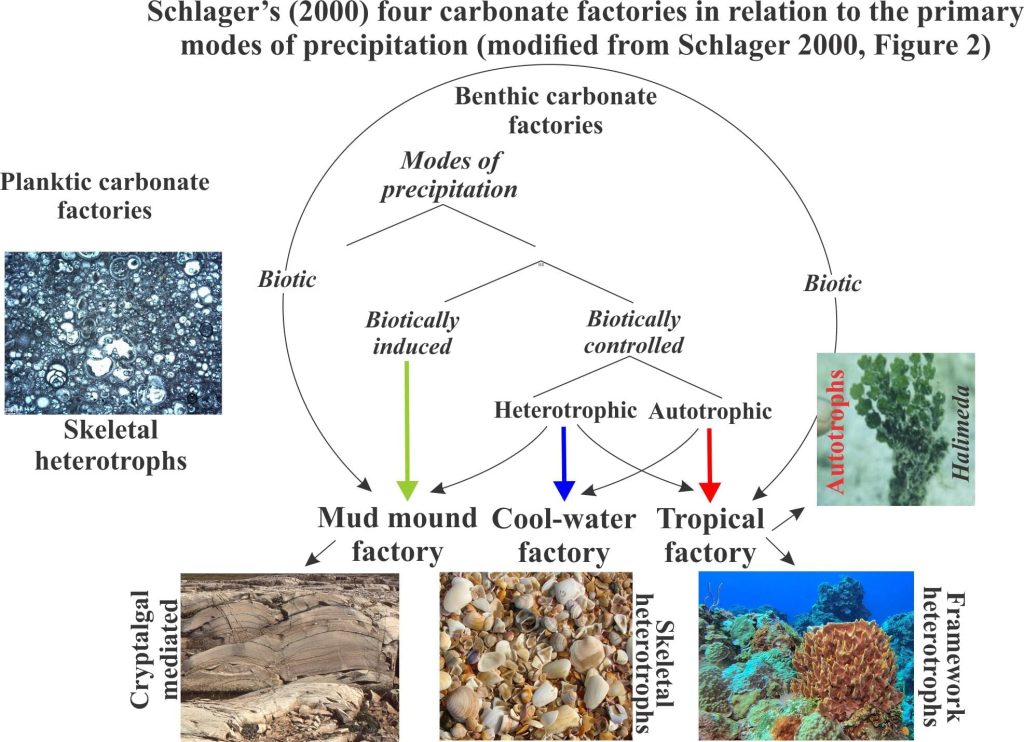
An important contribution to the factory concept was published by Wolfgang Schlager (2000) who identified three benthic factories plus a planktic realm. A modified version of Schlager’s carbonate production scheme is shown here.
Tropical factories occupy shallow, warm waters where the primary environmental determinants are temperature and sunlight; biotic processes dominate. This factory is limited to the photic zone. Photosynthetic algae (photoautotrophs) play a critical supply role, either as primary producers or as mediators for heterotrophic producers like corals (their symbiotic algae). Invertebrates like molluscs and bryozoans also play an important but subordinate role.
Abiotic and biotically mediated precipitation is a significant component of the Tropical Factory – ooid shoals form platform-wide subaqueous bars and dunes, and whitings form extensive blankets of lime mud (the debate between biotic and abiotic processes already noted). Seafloor cementation is abiotically controlled and dominated by relatively rapid precipitation of aragonite and high-Mg calcite cements. Seafloor cements form in a range of water depths, from the intertidal zone to outer platform and slope. Intertidal cementation is commonly manifested as beachrock that can form in a matter of months.
Juxtaposition of Tropical and Cool-water factories is also possible. This can occur where cool-water biotas become dominant at depths and water temperatures where tropical biotas do not thrive, particularly below the thermocline.
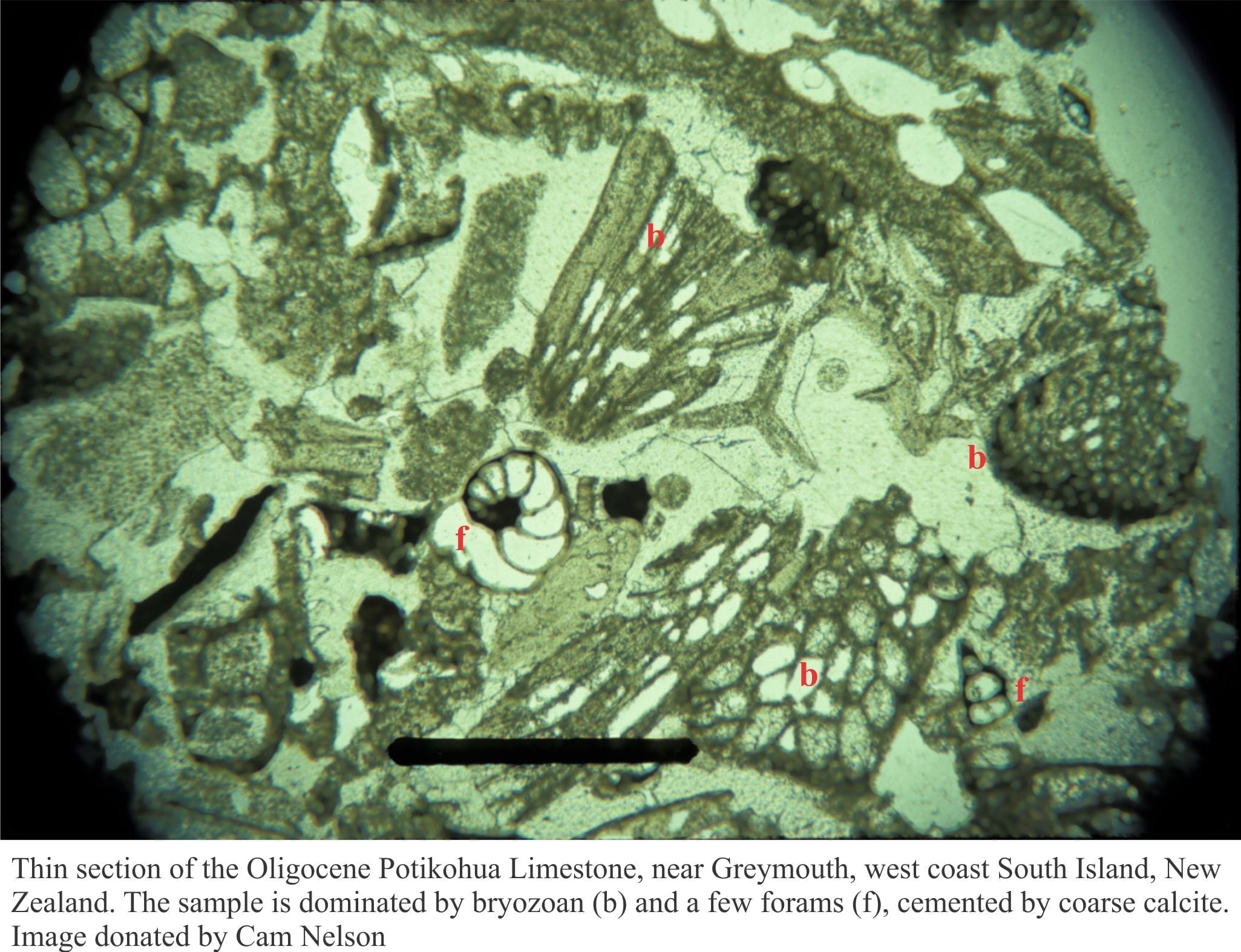
Cool-water factories in modern oceans extend from about 30o latitude to the polar regions, and at lower latitudes below the thermocline. They are dominated by heterotrophic biotas and can therefore extend to much greater water depths than their Tropical counterparts. Cool-water factories within the photic zone can also produce quantities of calcareous red algae. Deposits tend to be dominated by fragmented molluscs, barnacles, echinoderms, bryozoans, calcareous algae and some solitary Scleractinia corals. Benthic foraminifera are also common. Most deposits are distributed hydrodynamically.
There are no coral reefs, but bryozoans and oysters may accumulate as buildups and banks; good examples of oyster buildups are presented in the Oligocene Te Kuiti Group (New Zealand).
Sea floor cementation is far less common than in Tropical factories and where it does occur is dominated by low-Mg calcite.
Mud mounds are dominated by micrite precipitated biotically and abiotically. The name applies to three-dimensional mounds and to platform-wide buildups. Precipitation is commonly mediated by algae, bacteria and cyanobacteria and therefore is restricted to the photic zone. There is a cohesiveness to this sediment that prevents hydrodynamic distribution across the sea floor. Evidence in the rock record also indicates that some cementation took place in concert with deposition. Although cohesive and lithified, mud mounds are susceptible to erosion by storms, a common product of which is intraclast conglomerate.
There are several celebrated examples in the Phanerozoic, but it was in the Precambrian that cryptalgal mud buildups produced an extraordinary array of structures – from simple three-dimensional laminated mounds, to complex branched stromatolites and platform-wide buildups having several metres of synoptic relief (relief on the growing cryptalgal mat surface).
Planktic factories, the fourth in Schlager’s scheme, produce carbonate muds and oozes via ocean water masses. The primary producers here are phytoplankton and zooplankton that secrete calcite or aragonite tests. Common examples include coccoliths and foraminifera. They tend to accumulate in deep water, on parts of the sea floor that are devoid of terrigenous sediment. One example, celebrated in song, is the Cretaceous chalks of Dover.
The four factories here are perhaps the most basic. There is no doubt that some or all of them could be split into subfactories if you are inclined to splitting. It is also the case that there can be significant variation within any of the four, for example where a shallow platform is subjected to submarine freshwater discharge (springs) such that seawater is diluted locally, or where the opposite occurs in hypersaline conditions. In both examples the saturation state of calcite and aragonite may change drastically such forcing changes in the composition of benthic communities and abiotic cements.
Other posts in this series
Mineralogy of carbonates; skeletal grains
Mineralogy of carbonates; non-skeletal grains
Mineralogy of carbonates; lime mud
Mineralogy of carbonates; classification
Mineralogy of carbonates; basic geochemistry
Mineralogy of carbonates; cements
Mineralogy of carbonates; diagenetic settings
Mineralogy of carbonates; sea floor diagenesis
Mineralogy of carbonates; Beachrock
Mineralogy of carbonates: Stromatolite reefs
Some Important contributions
James, N.P., and Kendall, A.C., 1992. Introduction to carbonate and evaporite facies models. In Walker, R. G., and James, N. P. (eds.), Facies Models – Response to Sea Level Change. St. John’s: Geological Society of Canada, pp. 265–275.
Reijmer, J.J.G. 2016. Carbonate Factories. In: Encyclopedia of Marine Geosciences, Springer
Schlager, W. 2000. Sedimentation rates and growth potential of tropical, cool-water, and mud mound systems. In. E. Insalaco, P.W.Skelton, and T.J.Palmer (eds.). Carbonate Platform Systems: Components and Interactions. Geological Society London, Special Publication 178, p. 217-227.
Tucker, M.E. and Wright, V.P. 1990. Carbonate Sedimentology. Blackwell Science.