An introduction to the formation and chemistry of evaporites
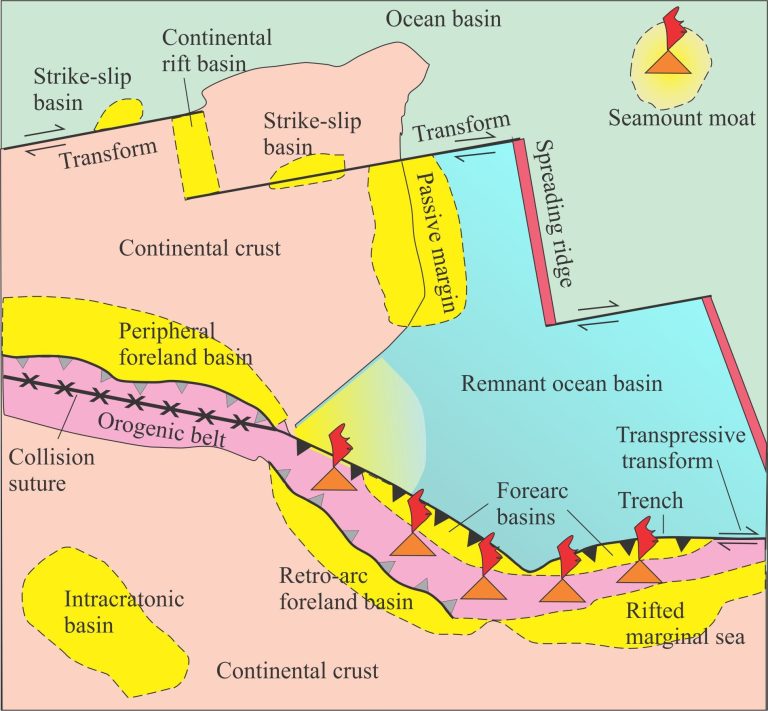
Evaporites form in arid climates, but their latitudinal extent is extremely broad – from the subtropics to polar regions. The starting point for marine evaporites is nearly always seawater. Those bound by terrestrial barriers, namely the saline lakes, can have hugely varied initial water compositions, and an equally impressive range of minerals. Marine evaporites require expansive seas that also have some kind of barrier to promote evaporation and prevent continuous mixing of new seawater. Perhaps the best known example is the Permian Zechstein Sea. There are no good modern analogues for marine basins of this extent.
Modern saline lakes on the other hand are numerous; iconic examples like Death Valley, Mono Lake, Lake Magadi, and the Dead Sea have been studied intensively. The critical condition for evaporites to accumulate is the balance between incoming water (precipitation, snow melt, groundwater seepage) and intense evaporation. Humidity is also a critical factor.
The posts that follow deal with some aspects of evaporite formation, beginning with those in saline lakes. This also provides the opportunity to examine some of the important literature from the 1960s – 70s, particularly the geochemical models and interpretations.
Mineralogy of evaporites: Saline lakes
Mineralogy of evaporites: Saline lake brines
Mineralogy of evaporites: Death Valley hydrology