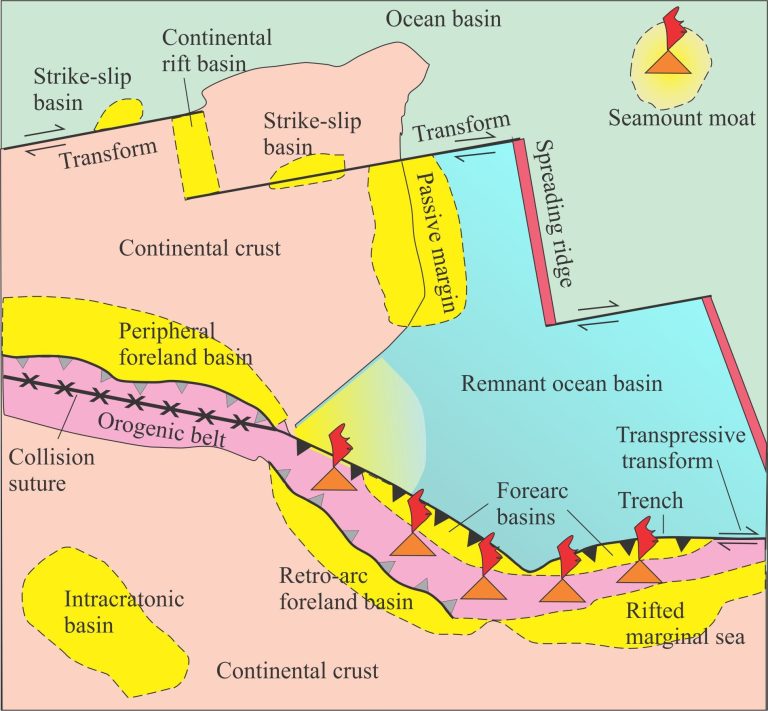
With the general emphasis on carbon emissions from fossil fuels and the ensuing discussions on climate change, we tend to forget some of the natural sources of greenhouse gases that continually leak carbon dioxide (CO2) and methane (CH4) into the oceans and atmosphere. Two such sources are gas hydrates beneath the sea floor and permafrost. Both sequester carbon, but the sequestration is rather tenuous; both can easily be disturbed by natural and anthropogenic processes.
The rate of warming in the Arctic, where most permafrost resides, and potential warming of shallow ocean waters have many scientists concerned about a possible snowball effect where melting could release significant quantities of CO2 and CH4. The amount of carbon stored in both these deposits is huge. In the permafrost alone there is an estimated 1400 giga (billion) tons of stored carbon; compare this with the approximately 850 gigatons of carbon currently in the atmosphere. What is poorly known is how much and how quickly this carbon might enter the atmosphere if wholesale melting were to occur. Large scale permafrost melting will also lead to an increase in freshwater runoff and seepage to the Arctic Ocean that, itself may influence energy transfer in ocean water masses.
Permafrost
Permafrost is defined as ground that remains frozen for 2 years or more. It is most mostly found in Arctic regions (North America, Siberia, Greenland) but also farther south in the Himalayas and parts of Mongolia. To put it into perspective, permafrost occurs beneath about 24% of the land surface of the northern hemisphere. It is also found offshore beneath the Arctic shelf. Permafrost may or may not contain ice; thicknesses up to 1000m have been recorded. Carbon, that has accumulated since the Last Glacial Maximum (about 20,000 years ago) is stored in the frozen soil and sediment. Under normal summer conditions some permafrost melts; melting may result in unstable ground where building foundations subside, or where landslides and mudflows develop from slope failure. However, accelerated warming in the Arctic over the last few decades has resulted in an increase in permafrost melt. Melting exposes the carbon in the soils to microbial activity; depending on the types of microbe these can produce CO2 or CH4 gas that, in turn may either be consumed by other microbes (and therefore sequestered), or released to the atmosphere. The ability of these thawed active soils to sequester carbon is still poorly understood.
Under normal summer conditions some permafrost melts; melting may result in unstable ground where building foundations subside, or where landslides and mudflows develop from slope failure. However, accelerated warming in the Arctic over the last few decades has resulted in an increase in permafrost melt. Melting exposes the carbon in the soils to microbial activity; depending on the types of microbe these can produce CO2 or CH4 gas that, in turn may either be consumed by other microbes (and therefore sequestered), or released to the atmosphere. The ability of these thawed active soils to sequester carbon is still poorly understood.
Methane beneath the sea floor
Under certain conditions of temperature and pressure, methane can be locked in an ice cage in layers beneath the sea floor. Methane hydrate (or methyl hydrate) is a solid, ice compound wherein a molecule of methane is surrounded by water molecules in a cage-like structure (also called a clathrate). There is no chemical bonding of the CH4 to the water; the CH4 is only weakly held in the cage and hence can easily break free if melting occurs. The methane is held in the water molecule cage partly by pressure, which means that the hydrates cannot exist as solids at depths shallower than 250-300m beneath the sea floor.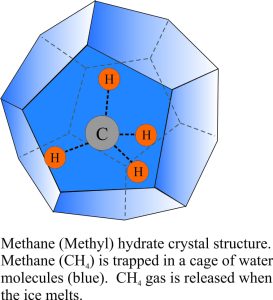 The methane is derived primarily from biogenic processes; it is a by-product of micro-organisms (e.g. bacteria) that consume organic matter (e.g. dead plankton) that has been buried beneath the sea floor. The gas, as it is produced moves through porous sediment towards the sea floor. En route it is converted from the gas to solid (ice) hydrate in sediment that is close to 0oC.
The methane is derived primarily from biogenic processes; it is a by-product of micro-organisms (e.g. bacteria) that consume organic matter (e.g. dead plankton) that has been buried beneath the sea floor. The gas, as it is produced moves through porous sediment towards the sea floor. En route it is converted from the gas to solid (ice) hydrate in sediment that is close to 0oC.
Evidence of gas hydrate layers just below the sea floor comes from two main lines of evidence; drill core and drill logs (that scientists can examine directly), and seismic surveys. The methane hydrate layers have much lower density than other mud and sand layers and show up on seismic profiles as a prominent reflection that has become known as a bottom-simulating reflection (BSR) (seismic surveys use sound, or acoustic energy to detect different layers of rock based on contrasts in their density). Methane hydrate layers have now been found beneath the ocean floor off many coasts around the Pacific, Atlantic, Arctic and Antarctic oceans. They are also found in some regions of deep land-bound permafrost. The total volume of methane gas locked into these hydrate layers must be absolutely huge – vastly greater than the volume of methane currently in the atmosphere.
Methane does escape the sea floor through vents. Methane flares, or bubbling and elevated methane concentrations have been observed at some of the many vents off the east coast of North Island, New Zealand, and recently have also been reported from the East Siberian Arctic coast; some of the Siberian submarine vents are more than 1000m in diameter. At the New Zealand sites, little if any of the methane reaches the sea surface. Here, most of the methane is oxidized by sulphate reducing bacteria that live in the sea floor sediment (producing HCO3– and HS). However, methane venting off the Siberian coast is voluminous enough to send the gas bubbling to the sea surface where it is released directly into the atmosphere.
Trends
Apparently the Arctic is warming significantly faster than the rest of the planet. An obvious manifestation of this trend is the depletion of sea ice, year on year. Changes to land-bound permafrost are clearly visible, especially when your house begins to subside into soggy tundra. Less visible are the changes to carbon chemistry as frozen soil thaws and soil microbes do their thing. Likewise, any warming of shallow (a few 100 metres) ocean water temperatures has the potential to create greater instability in gas hydrate layers beneath the sea floor. From a purely scientific point of view we still don’t understand how some of these melting-gas release processes will play out. For example, at what point will the natural microbiotas, that consume a fair bit of the released CO2 and CH4, be overwhelmed by a flood of gas. A recent article in Nature Communications suggests that the rate of methane release from gas hydrates buried beneath the Barents and Norwegian seas, since deglaciation (~17 thousand years ago at these sites) was protracted rather than as massive bursts. Whether the same rate of gas release will continue in the present climate conditions is not yet know. Science will eventually work this out, but let’s hope it’s sooner rather than later.