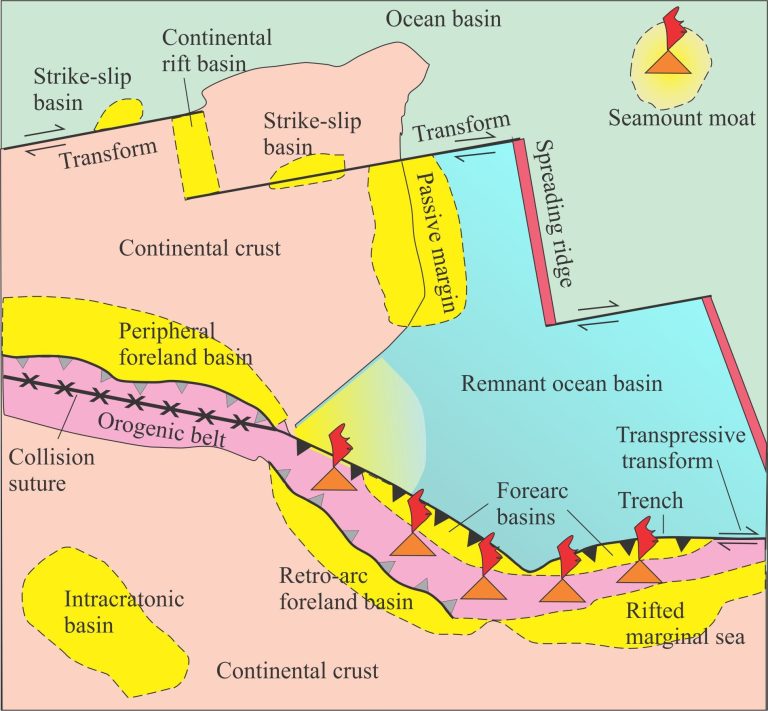
See also the companion Glossary of Petrography and Petrology
Absolute zero: The theoretical temperature, measured in degrees Kelvin (K), where the kinetic energy of atomic particles is zero – everything is at complete rest. The temperature value corresponds to -273.15o C and -459.67o F. Thus, 0o C is 273.15o K.
Abiotic: Physical and chemical conditions not directly associated with life forms, but interact with biotic conditions to form ecosystems. For example, salinity, pH, temperature, precipitation. The term includes organic compounds present in abiotic conditions such as comets. Cf. prebiotic.
Absorption: The process where a chemical component (ion, molecule) enters the atomic or molecular framework of a different substance. For example, strontium ions (Sr2+) can be absorbed into the aragonite (CaCO3) crystal lattice. Cf. Adsorption.
Acid: A substance that releases or donates a proton when dissolved in water. The proton is a hydrogen ion that in solution associates with an H20 molecule to form H30+ , but is usually written as H+ . Acids react with bases (bases contain hydroxyl ions – OH– ). Water may act as an acid or a base. Solutions with excess H+ are acidic, such that pH < 7.
Acid dissociation constant (Ka): Equivalent to the equilibrium constant, and calculated from the acid dissociation equilibria. For example, HCl dissociates completely (strong acid) and hence there is one reaction and one value of K. HCl + H2O ⇄ Cl– + H3O+.
KHCl = [Cl–].[ H3O+]/[HCl] Also written as log values pKHCl = Log KHCl
Acid rain: Normal rain has a pH of 5 – 5.5. Acid rain is pH <5, and usually pH 4 or less. It occurs when there is excess SO2 and NO2 in the atmosphere, mostly from industrial sources, but can also occur during volcanic eruptions and the formation of sulphuric acid aerosols.
Activation energy: The minimum energy for a particular reactant to form a new product in a chemical reaction. Calculation of the activation energy is a critical step to understanding reaction pathways and rates in chemical kinetics. The AE is commonly associated with a chemical state transitional between the reactant and product compositions. It is usually expressed in the Arrhenius equation as a function of reaction rate.
Activity (geochemical): Sometimes referred to as effective concentration. The activity of an ion is the ratio of its concentration versus some standard concentration and is therefore dimensionless (unlike concentration). The ratio is calculated using an activity coefficient. It is used in equilibria because it expresses the amount of an anion or cation that is available for reaction; compare concentration that measures the total amount of an ion. In a solution like sea water there are many different cations and anions, all reacting to collisions of various kinds. For example, the CO32- anion may collide with cations other than Ca2+ (Na+, Mg2+, K+ and so on), such that the amount of CO32- available to react with Ca2+ is less than the measured concentration. In other words, the amount of CO32- available in real solutions depends not just on its overall concentration, but also on its environment. For this reason, it is preferable to use activities in thermodynamic calculations, such as equilibrium constants. The activity of solids is usually taken as 1.
Activity coefficient: The activity coefficient (γ) for a specific ion species is related to the degree of ionic interaction with other species in solution. For dilute solutions γ approaches 1 because there are few ion interactions (γ is dimensionless). Thus, the γ value for HCO3– in fresh river water averages about 0.95, but in sea water is much lower (0.57) because of ionic interactions. Activity (a) is calculated for specific ions from the relationship:
a = γ m where m is concentration.
Adiabatic process: A thermodynamic process in which no heat is transferred to or released from the system during mechanical work – like expansion or compression. It is usually accompanied by a change in temperature – expansion of gas/fluid causes a fall in temperature. It is a reversible process. Cf. Isothermal process
Adhesion: The tendency for particles or surfaces of different structure or composition to adhere – cf. cohesion is where similar particles or surfaces adhere.
Adsorption: The process where an atom, ion or molecule adheres to the surface of another substance – it does not become part of the substrate chemical make-up. Cf. Absorption.
Aeolianite: Dune sands cemented by calcite are an example of shallow meteoric-vadose zone diagenesis. Dune sand mineralogy may be siliciclastic or bioclastic, or a mix of both. Most common in subtropical to tropical coastal dunes.
Aerobic conditions: Reactions that directly utilise available oxygen, the most obvious being respiration in life forms, where oxygen is used in metabolic reactions to generate energy (e.g., from food). In sediments this generally is associated with the metabolic activity of microbes – a distinction is made between these types of reaction and oxidation reactions that do not require intermediary metabolic activity in life forms. Cf. Anaerobic conditions.
Aerosol: Dispersions of liquid or solid particles in a gas. For example, water vapour, smoke (solid particles). Aerosols of sulphuric acid are commonly produced from explosive volcanic eruption columns that project through the troposphere (sulphur dioxide and water).
Alizarin Red-S: This is a soluble organic acid that reacts with calcium. Distinguish between calcite (stains pink-red) and dolomite (no stain) can be easily done using this stain, on rock slabs or thin sections.
Alkali metal: Metals in Group 1 of the Periodic Table, that include lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), and Rancium (Fr).
Alkali Earth Metal: Metals in Group 2 of the Periodic Table: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
Alkaline: An aqueous solution having a pH > 7
Alkalinity: Alkalinity is a measure of the amount of acid that can be added to an aqueous solution without causing significant changes to the pH; also referred to as the acid neutralizing capacity or buffering capacity. The total alkalinity of seawater is primarily determined by the major anions:
mHCO3– + 2mCO32- + minor constituents like borate, phosphate, and silicate anions.
Ångström: (Å) A unit of length – 10-10 m. cf. A nanometre is 10-9 m.
Anhydrous: A mineral form lacking water of crystallization. For example, anhydrite (CaSO4) is anhydrous and the hydrous form gypsum is (CaSO4·2H2O).
Alpha particle: A high energy particle consisting of two protons and two neutrons emitted from a nucleus during radioactive decay. The particle has the same composition as a helium-4 nucleus. The name was coined by Earnest Rutherford during his studies of uranium decay.
Amino acid: The building blocks of proteins – all proteins contain up to 20 amino acids. All have the same basic structure – a central carbon atom bonded to four groups: Hydrogen, a carboxyl group (the general formula COOH where C=O is a double bond, plus C-OH), an amine group (derivatives of ammonia – NH3) and an R group (more complex side chains, some of which are soluble in water). The amino acid glycine has been found in comets and asteroids.
Anaerobic conditions: conditions where metabolic reactions in life forms do not require molecular oxygen. In sediments, such reactions are commonly generated by microbes that reduce oxygen-bearing compounds like sulphate (to sulphide), nitrate (to nitrite or ammonia), and carbon dioxide to methane. Sediment where these conditions persist tend to be green-black, and may have mineral sulphides (e.g., iron, manganese). One example is the sediment beneath wetlands, including marginal marine mangrove wetlands. Cf. aerobic conditions.
Anhydrite: Ca SO4. Colourless, orthorhombic, biaxial (+), low relief in thin section. Good cleavage at (100), (010), and (001); cleavage traces are orthogonal, cleavage fragments commonly show cubic terminations and indentations. Extinction parallel to cleavage traces. Twin lamellae intersect at about 90o. Primarily a sedimentary chemical precipitate at surface temperatures, for example in sabkhas. A common component of evaporite deposits, with halite, gypsum, and sylvite. Also associate with calcite or dolomite.
Anion: A negatively charged ion; an atom that that has gained one or more electrons such that there is a charge imbalance between the nucleus (protons) and electrons.
Anode: A negative electrode that attracts positive aqueous ions.
Anoxic conditions: Usually applied to aqueous environments (water masses as well as connate water) where there is none, or insufficient dissolved oxygen for respiration; usually measured at less than 0.5 ml/L. Under these conditions, the sources of oxygen via bacterial reduction are from nitrates and sulphates. Once these sources are depleted carbon dioxide becomes an important source during reduction to methane. Deep waters in lakes where there is no turnover of the water mass, can become anoxic. Anoxia are also implicated in some of Earth’s major extinctions, such as the Late Permian – Triassic event. Early Precambrian oceans and lakes were probably anoxic. e.g., März and Brumsack, 2015.
Aragonite: CaCO3. A polymorph with calcite, and distinguished from the latter by its orthorhombic crystal form, usually fibrous needle or acicular habit, commonly as radial clusters in cement fill, biaxial (-) (calcite is uniaxial (-)). Aragonite lacks rhombohedral cleavage. It is a common mineral in geologically recent carbonate and evaporite deposits and invertebrate shells but tends to recrystallize to low magnesium calcite during burial. Also as a hydrothermal precipitate.
Aromatic compound: An organic, carbon ring, usually 6 or 5-sided, with alternating (conjugated) single and double bonds. Benzene and toluene are common examples. Compounds may have a single ring or several covalent bonded rings.
Atmosphere (pressure): An alternative unit to the SI standard. One atmosphere (atm) value is the average air pressure at mean sea level measured as 760 mm (29.92 inches) of mercury. It is equivalent to 101,325 Pa, 1,013.25 millibars, and 14.692 psi. (pounds per square inch).
Atomic number: In ordinary atom nuclei it is the number of protons. If the atom is not charged (e.g. an ion) then the atomic number also equals the number of electrons (because of charge balance with the nucleus). Isotopes have the same atomic number but different numbers of neutrons.
Avogadro’s Constant (Avogadro’s Number): The number of atoms or molecules in one mole of a pure substance (an element or compound) – 6.022 x 1023. Named after Italian chemist Lorenzo Avogadro (1776-1856).
Avogadro’s Law: Equal volumes of gases under the same temperatures and pressures will contain equal numbers of molecules. Hypothesized in 1811 and named after Italian chemist Lorenzo Avogadro (1776-1856).
Base: A base is a substance that gains a proton in aqueous solution. This can be written in a generalized way as H+ + OH– ⇄ H20. Water can act as a base or an acid. Solutions with excess OH– are basic with pH > 7.
Berzelius, Jöns Jacob (1779-1848) The Swedish chemist who devised the one and two letter abbreviations that we use today for the chemical elements (O – oxygen; Fe iron etc.). He also discovered Cerium, Selenium, Silicon, Thorium, and isolated Zirconium.
Beta particle: A high energy electron or positron emitted from a nucleus during radioactive decay.
Biogenic gas: Natural gases produced by microbial breakdown of organic compounds usually at temperature <50oC and most commonly at depths of a few 10s of metres (although this can extend to a few 100 metres). Methane comprises 99% of biogenic gas. Biogenic gas is also found with coal deposits up to high volatile bituminous rank. It is also produced in peat bogs and garbage landfill sites. It is possible to distinguish biogenic from thermogenic gas using stable C and deuterium isotopes.
Biomould: Also biomold. The impression of an organism left in a rock following dissolution of the original skeletal mineral – commonly calcite and aragonite. A cast of this impression is formed if the mould is filled with a new precipitate or sediment.
Biosignatures: Chemical compounds and elements that are the products of living metabolic processes and that cannot be synthesized by abiotic processes. For example, molecular oxygen, carbon dioxide, and methane are well known bioproducts but they can also be produced by non-biological processes. Many organic compounds are potential biosignature types although some amino acids have been detected on comets and meteorites (e.g., glutamic acid, glycine, and some aspartic acid, serine, alanine, β-alanine, and γ-amino-n-butyric acid) and amines.
Bitumen: A very viscous, black hydrocarbon, liquid or semi-solid. It is the final product during oil fractionation – natural or industrial. It contains free carbon. Commonly used as a sealant on roads and construction sites.
Boiling point: The temperature at which there is a phase change from liquid to vapour or gas. It is also dependent on the vapour pressure of the substance, for example water will boil at 100oC at sea level, and 83.3o C at 5000 m a.s.l.
Botryoidal cement: In limestones, this cement form is presented as radial clusters of fibrous or bladed calcite or aragonite that precipitate in more cavernous porosity. Common examples are found in reef frameworks, and fenestrae that form by mineral dissolution, gas bubbles, and crystal expansion (e.g. halite-gypsum crystal growth in sabkhas). Fenestrae are common in some cryptalgal laminates and mud mounds containing Stromatactis.
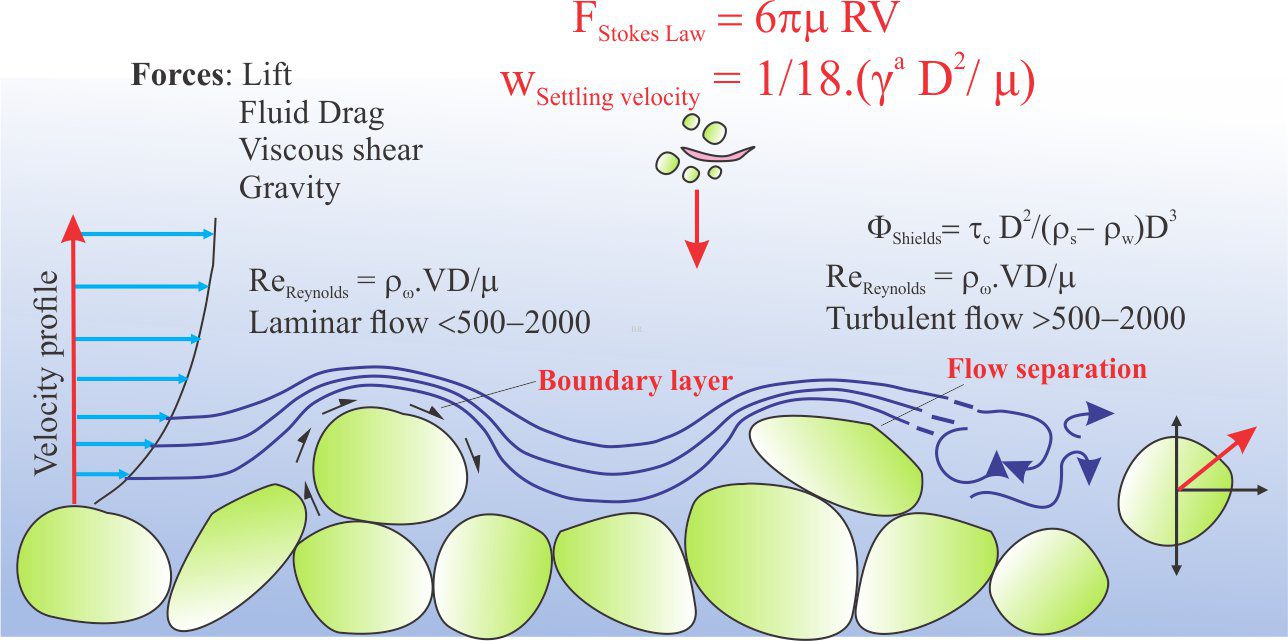
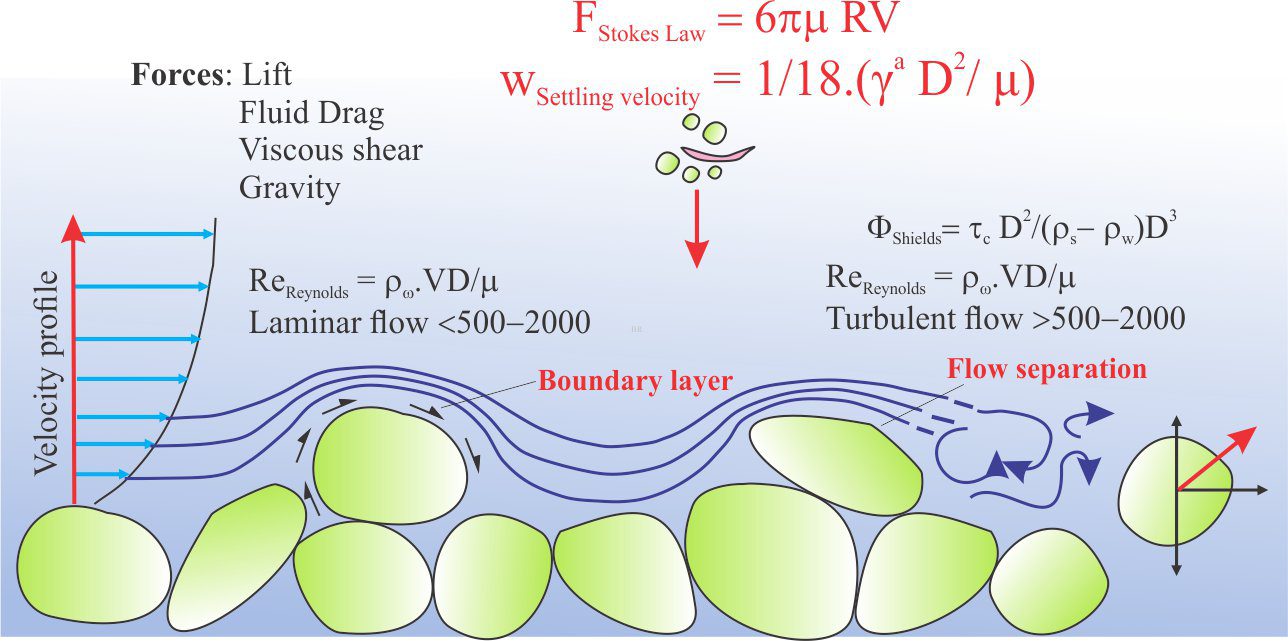
Boundary layer: A thin layer of fluid in contact with a solid surface, usually in the context of fluid flow. Flow velocities are reduced by friction and depending on surface roughness particle drag. Depends mainly on fluid viscosity and temperature. Flow in boundary layers generally have low Reynolds numbers indicating laminar flow.
Brines: Generally used for natural waters more saline than seawater. The main dissolved salt is sodium chloride (NaCl), but calcium and magnesium sulphates are also important constituents, and there are several important trace elements, such as lithium. The primary mechanism for brine concentration in ocean basins and saline lakes is evaporation. The saturation level for NaCl is about 357 ppt (normal seawater is 32 ppt).
Buffer: An aqueous solution that can resist pH changes when small amounts of acid or base are added. The buffer solution must contain a weak acid or weak base. An important buffer system necessary for life is the buffering of seawater by carbonic acid (H2CO3) that dissociates into several equilibria involving CO2 (gas and aqueous), CO32-, HCO3–, H2O, and H+ (or H3O+).
Calcite: CaCO3. Hexagonal-rhombohedral, polymorphous with aragonite. Uniaxial (-), excellent (1011) cleavage. Twinning common; in thin section twin lamellae tend to parallel the long diagonal of crystal rhombs. In thin section, it has high relief (RI 1.48-1.66), and high birefringence that creates a kind of twinkling appearance in thin section. Rare as a primary mineral in igneous rock (except in carbonatites), but does occur as an alteration product, and as vesicle filling; also common in hydrothermally altered rocks and geothermal precipitates. Common in many calcareous metamorphic rocks at amphibolite grade, and marble. Occurs in many guises in sedimentary rocks, as a primary constituent of skeletal and shelly fossils, as cements, and as matrix or framework replacement. Cement precipitates form euhedral rhombohedral or scalenohedral crystals; replacement precipitates tend to be anhedral. Magnesium replaces calcium up to 19% in some invertebrate shells. Distinguished from dolomite by one set of twin lamellae (not always obvious); dolomite commonly has turbid cores.
Calcite compensation depth (CCD): As ocean water depths increase, the partial pressure of CO2 increases and the temperature decreases – in both cases CaCO3 becomes increasingly soluble. An important consequence of this convergence is a decrease in CaCO3 saturation to the point where calcite and aragonite begin to dissolve. For calcite, the depths range from about 4.6 to 5.1 km. Aragonite is more soluble and the ACD depths are about 3 km. This means that the sea floor at or below these depth limits will tend to be devoid of calcareous sediment (particularly microfossils like foraminifera and coccoliths).
Calcite divide (geochemistry): The stage during evaporation of brines where calcite precipitation determines the succession of minerals in waters subsequently depleted in Ca2+ and CO32-. It determines whether the brine subsequently evolves as HCO3 rich or HCO3 poor.
Caliche: Also called calcrete. Soil horizons in which carbonate precipitation results in a hardened crust. They develop in regions in which evaporation exceed precipitation, where periods of wetting alternate with drying. Thus, carbonate textures commonly show evidence of dissolution and reprecipitation. A common product is vadose pisoids that also show evidence of multiple episodes of dissolution and precipitation. They can develop in alluvial-lacustrine and intertidal-supratidal settings.
Capillary zone: In hydrogeology, also called the capillary fringe. It is a relatively narrow interval above the watertable where surface tension forces on aquifer materials cause water to rise and partly fill pore spaces. The capillary fringe is part of the unsaturated, or vadose zone.
Carbonates: The most diverse group of sediments and sedimentary rocks, usually presented as limestones and dolostones. Carbonate precipitation (and dissolution) is based on the chemical equilibria involving CO2, HCO3–, CO32-, and H2CO3. Their primary mineralogy includes calcite and aragonite polymorphs (CaCO3), and dolomite (Ca.Mg [CO3]2). Carbonate formation at Earth’s surface is intimately associated with biological production where precipitation is either induced directly by organisms, or indirectly promoted by the activity and metabolism of organisms. Organisms involved in carbonate production range from microbial to large invertebrates.
Carbonic acid: A weak acid that forms naturally from the reaction:
CO2 + H2O ⇄ H2CO3
It is the primary cause of slight acidity of rain (pH 5.5 to 5.8). It is an important component in the series of carbonate equilibria, particularly for pH buffering.
Catalyst: An element of compound that increases the rate of a reaction or enables the reaction to approach its activation energy, without being consumed in the final products. Enzymes are important catalysts in a large number of biological reactions.
Cathode: A positive electrode to which negative aqueous ions migrate.
Cation: A positively charged ion; an atom that that has lost one or more electrons such that there is a charge imbalance between the nucleus (protons) and electrons.
Celsius: The temperature scale where freezing point is 0o C and boiling point 100o C at atmospheric pressure, the scale range being divided into 100 equal points. The word is derived from Latin centum. The scale was devised by Anders Celsius in 1742. He originally named it centigrade, but in 1942 the International Committee for Weights sand Measures renamed it in his honour. The link takes you to a history of Celsius.
Celsius-Fahrenheit conversion: (The value in °C × 9/5) + 32 = °F. The opposite conversion is the (value of °F – 32) × 5/9 = °C).
Cement: Precipitation of pore-filling minerals, such as quartz, calcite, aragonite, high-magnesium calcite, dolomite, clays, and gypsum, is an important process during sediment lithification. Crystal growth begins at grain boundaries, gradually filling the available pore space. Cementation can begin at the sea floor, particularly by aragonite and calcite, and continue during burial. Cementation gradually occludes effective porosity. Distinguishing cement and neomorphic textures, see Neomorphic textures in thin section
Chalcedony: A fibrous form of microcrystalline quartz, or chert. It commonly form radial clusters. Under crossed polars, extinction patterns are sweeping or radial.
Charge number: Expresses the number of electrons (as a superscript) that are deficient (e.g., Ca2+) or in excess for an ion (e.g., CO32-).
Chemical equilibria: Chemical reactions normally written with the reactants on the left and products on the right. The two are separated by either:
- An equal sign indicating equilibrium, where forward reactions (to the right) equal reverse reactions, or
- By two opposing arrows that indicate forward and reverse reactions (⇄).
Equilibria should be charge and mass balanced. The quantities of reactants and products are written as concentrations or activities.
Chemical equilibrium: At equilibrium there is no net gain or loss of reactants (by convention, the left side of the equation) or products and no net change in energy. Note that this does not mean the system is static – even at equilibrium there are still collisions between ions (all reactions in solution involve collisions), but collisions on the left equal those on the right side of the equation.
Chemical facies: (hydrogeology) This is a useful concept to demonstrate the chemistry of groundwater in relation to aquifer rock-sediment composition, and the evolution of groundwater chemistry as it flows from one rock type to another. For example, flow from sandstone to limestone aquifers will be accompanied by a change in HCO3– and pH, plus the concentrations of cations like calcium and magnesium.
Chemical kinetics: Also called Reaction kinetics. This is the study of reaction rates and reaction pathways, and hence is distinct from thermodynamics that deals with energy transfer during reactions and is independent of rate. Kinetics is a measure of the rate of change (in concentration or activity) of both reactants and products, in reversible and irreversible reactions. It is particularly important in reactions that are slow relative to mass/solute transport. A good example if these conditions is the precipitation of dolomite under surface conditions – the reaction is thermodynamically favoured, but kinetically is very slow. Kinetics is related to thermodynamics in terms of equilibrium constants, the activation energy of reactions (i.e. Gibbs free energy), and temperature. As a general rule, the rate of 1st-order reactions doubles for every 10º increase in temperature.
Chemical potential: This measure of the potential for chemical work in a reactive system and is usually expressed as the partial derivative of the change in Free energy if you add or subtract material (particles) from the system, at constant temperature and pressure. It is usually written: μ = ∂G/∂ni where ni is the number of particles of a certain type (atoms, ion, molecules). Because ni is dimensionless, μ has units of energy.
Chemical stability (of sedimentary grains): The ability to resist dissolution or chemical change during sediment transport and burial. Quartz tends to be chemically stable, compared with feldspar that may react, particularly during burial diagenesis to form new minerals such as clays. Minerals like zircon are extremely stable and can survive several sedimentary cycles.
Chemocline: A boundary within a water column at which there is a fairly abrupt change in chemical gradient. Examples include the boundary between fresh water and seawater, or changes in REDOX conditions, from oxidation to reducing.
Chemotroph: Organisms that obtain their metabolic energy and synthesize biomass (such as carbohydrates) from reduced elements like sulphur, sulphide, and ferrous iron, instead of sunlight.
Chitin: One of the most important biological polymers in nature, it consists of polysaccharides and forms a tough, insoluble exoskeleton for insects, crustaceans, and arachnids, and some parts of molluscs like gastropod radulae and cephalopod beaks.
Chlorite: has low birefringence, in varying shades of green (in PPL), and crystal habit that is also variable, from fibrous, spherulitic or vermiform (worm-like). May be pleochroic in shades of green and yellow. It is commonly associated with low grade metamorphism and hydrothermal alteration. In greywackes and other mud rocks it is a common replacement for clay matrix, micas, and ferromagnesian minerals.
Clathrate: A general term for gas molecules that become trapped in an ice crystal cage. There are no chemical bonds between the gas and water ice and the gas can be released upon melting. Also called gas hydrates. Vast amounts of methane are trapped this way beneath the sea floor and in permafrost.
Cleavage: A plane of weakness within a crystal that will break with relative ease. It is caused by weak bonds between planes of atoms within a crystal lattice; the pattern of weakness repeats regularly through a crystal. Some minerals have poor or no cleavage (e.g., quartz, olivine); others have excellent cleavage along several lattice planes (e.g., calcite, feldspar). Cleavage is a defining characteristic of a mineral, particularly in thin section.
Closed system: In thermodynamics a system in which reactions or work can be done but where there is no transfer of energy or force beyond the system boundaries, i.e., there is no transfer of angular or linear momentum, energy, or mass – all these conditions remain constant within the system. It is an ideal system.
Cohesion: Cohesion: The tendency for particles or surfaces of similar composition or structure to adhere = cf. adhesion involves different kinds of particles.
Compaction: The process where sediment particles, once deposited, are pushed closer together to form a more tightly knit framework. Compaction begins almost immediately following deposition and continues during sediment burial. The normal compressive stress in this case is applied by the overlying sediment. Because porosity is also reduced, an additional requirement for compaction to take place is the release of interstitial water through aquifers. If fluid cannot escape (for example because of permeability barriers) then the rock body will not compact, and internal fluid pressures will rise – this is called overpressure. Mudrocks can compact to less than a tenth their depositional thickness. More rigid frameworks like sandstones compact far less. See also pressure solution, lithic fragments.
Component: In the context of Gibb’s Phase Rule, a component is the minimum number of chemicals (aqueous ions, molecules) that combined will make up the chemical species in the equilibrium system under consideration. For example, the equilibria CaCO3(s) = Ca2+ + CO32- has three species but two components – either CaCO3(s) and Ca2+ or CaCO3(s) and CO32-. In each case the third species can be made from combinations of the other two. In this example there are two phases.
Conjugated compounds: In organic chemistry, it describes the alternation of single and double covalent bonds. Typical examples are found in carbon ring compounds like benzene.
Covalent bond: The link between two atoms where electron pairs are shared. Cf. Ionic bond. Covalent compounds tend to be stable and less soluble in water than ionic compounds. Most organic compounds involving C-C and C-H paring are covalent.
Crude Oil: Oil of any composition as it is produced from a well or seep.
Crystallographic axes: Three or four axes about which a crystal can be rotated through 360o. The axes intersect at a single point (the centre of symmetry). They are labelled according to their lengths. If axes are the same length, then they are referred to as a1, a2, a3 etc. If they have different lengths, they are labelled a, b, and c. Thus, in the cubic (isometric) crystal system they are labelled a1, a2, a3, and in the tetragonal system a1, a2, c. The hexagonal system is the only one with four axes. Angles between axes are labelled α, β, γ.
Crystal overgrowths: A term usually reserved for diagenetic textures in rock cements, where a new crystal overlies, or overgrows the detrital mineral. The overgrowth may be the same composition and in crystallographic and optical continuity with its parent grain (this is a syntaxial overgrowth) as is common in quartz arenites, or different composition and unrelated crystallographically (epitaxial overgrowths).
Crystal symmetry: Symmetry describes the shape of an object and can be represented both mathematically and visually. In crystallography, the two most useful forms of symmetry are (mainly because they are the easiest to visualize):
- Axes of rotation (crystallographic axes) where a particular crystal face will be repeated during rotation through 360o. The number of repetitions for a 360o rotation can be 2, 3, 4, or 6, that are referred to as two-fold, three-fold, four-fold, and six-fold (axial) symmetry respectively.
- Planes of symmetry where two parts of a crystal are mirror images. For an analogy, think of this concept in terms of the common bilateral symmetry in many living organisms, such as people, and many classes of mollusc. Note that planes of symmetry are NOT the same as twin planes.
- Additional elements of symmetry include: A centre of symmetry, where a crystal face is reflected from one side to another or is repeated by inversion, and an axis of rotary inversion.
Crystal systems: There are 6 crystal systems based on combination of the elements of symmetry; a seventh system – trigonal – is usually considered a subclass of the hexagonal system. There are 32 crystal classes based on combinations of the symmetry elements. The defining criteria are axial lengths, the angles between axes, and axial symmetry (the number of repetitions about an axis).
Cubic (or Isometric) crystal system: The most symmetric group. All three axes are the same length and are at right angles to each other.
a1 = a2 = a3 α = β = γ = 90o
2, 3, and 4-fold symmetry depending on the class
Common crystal forms: cubes, octahedra, dodecahedra.e.g., Halite, pyrite, fluorite, garnet.
Decay series: The term applies to radionuclides with two or more decay products. Radionuclide decay will continue until a stable atomic structure is reached. Each radionuclide in the series has a half life. For example, unstable 40K decays to two paths – the most common is 40Ca, and least common 40Ar; the K-Ar series has a half life of 1.251 billion years.
Deionized water: Water that has had soluble ionic compounds removed, usually by ion-exchange resins or filters. It is used in wet chemical experiments and analyses. Cf. Distilled water.
Deliquescent materials: Hygroscopic minerals that absorb enough water from their surroundings (usually air) to completely dissolve them. Halite is a good example.
Diagenesis: The sum of physical and chemical processes in sediment, beginning soon after deposition at or immediately below the sediment-water interface, and continuing at depth in concert with increased burial temperatures, lithostatic and hydrostatic pressures, and changing fluid composition.
Dispersion: In geofluids this is the process where dissolved and insoluble compounds move from their source or point of origin; observed in groundwater flow, diagenesis, and metamorphism. In these contexts there are two primary mechanisms – mechanical dispersion, and molecular diffusion.
Dissequilibrium compaction: Under normal conditions of compaction, fluid that is driven from pore spaces escapes without a significant increase in pore pressure – i.e. hydrostatic conditions prevail. However, rapid deposition of low permeability deposits can impede fluid flow and under these conditions pore pressures increase; this process is called disequilibrium compaction. In many basins, this occurs at about 3km burial depths. Disequilibrium compaction is enhanced by cementation and tectonic compression.
Dissociation: Dissolution of an ionic compound in water producing cations and anions. For example, one molecule solid halite (NaCl) dissociates to one Na+ cation and one Cl– anion – each ion is weakly bound by an envelope of water molecules. Dolomite (CaMg(CO3)2 on the other hand dissociates to Ca2+, Mg2+, and two (CO32-) anions, written as
CaMg(CO3)2 ⇄ Ca2+aq + Mg2+aq + 2(CO32-)aq
Dissociation of water: The splitting of water into H+ and OH– ions, a reversible reaction written as
H2O ⇄ H+ + OH–
In most cases, the activity of water is one, so the equilibrium constant is written as
Kw = (H+) (OH–), which at 25oC is Kw = 10-14.
H+ ions on their own are very unstable, and combine with a water molecule to form H3O+, H5O2+, and H7O3+. Thus the notation H+ is shorthand for any of these ions.
Distilled water: Water purified by separation of the vapour condensate from a boiling liquid. Cf. deionized water.
Dolomite: CaMg(CO3)2. In highly ordered dolomite the unit cell lattice contains Ca and Mg layers that alternate separately with carbonate layers – it is sometimes referred to as a double salt. The atomic arrangement differs from magnesium calcite where the Mg ions are more or less randomly replace Ca in the unit cell lattice. Many sedimentary dolomites are calcium-rich. Hexagonal (Trigonal) rhombohedral crystals, uniaxial (-) with high relief, high birefringence that imparts a characteristic twinkling in thin section. Unlike calcite, twinning is not common but where it does occur twin lamellae parallel both long and short diagonals in rhombohedra. Most commonly a sedimentary mineral, replacing calcite, high magnesium calcite, and aragonite, but it can also form primary cements during sediment burial. Also occurs as a contact metamorphic and hydrothermal alteration phase.
Dry ice: Solid carbon dioxide – the freezing temperature is −78 °C (−109 °F). Dry ice sublimates to CO2 gas (there is no liquid phase at standard temperatures and pressures.
Drusy cement: Cements consisting of calcite rhomb mosaics that line and fill pores, intraskeletal chambers, and more cavernous porosity. The size of calcite rhombs commonly increases towards the center of void spaces. Intercrystalline boundaries tend to be planar. They are common in meteoric and burial environments where they may overlie earlier fibrous or bladed cements.
Dry gas: Reservoir gas composed primarily of methane (CH4) with minor amounts of ethane, propane and butane. Thermogenic dry gas forms at the high temperature end of the oil-gas window.
Electrode potential: Defined as the electrical potential (voltage) between an electrode and a standard hydrogen electrode (the anode). Reactions at the cathode involve reduction; at an anode oxidation. A complete electrochemical cell will have an electrode and an anode – each will have a potential independent of the other. The cell potential is the difference: Ecell = Ecathode – Eanode
Electrolyte: A compound that when melted or dissolved in water dissociates into ions capable of transmitting an electrical current. The current results in transfer of mass – negative ions to the positive electrode (cathode) and positive ions to the anode.
Electron: A negatively charged elementary atomic particle with a mass 1/1837 of a proton. Electrons surround an atomic nucleus but their shape and orbits are different for each element. It is a quantum object that acts both as a particle and a wave. It is never stationary. Electrons are not composed of other particles – they are fundamental.
Endothermic: A chemical reaction that absorbs energy from its surroundings, usually as heat. Common examples include photosynthesis, and evaporation of liquids.
Enthalpy: Enthalpy (H) describes the thermodynamic potential of a system to do work – it is also called heat content. It is usually calculated as the change in Enthalpy from one state to another. The heat absorbed or released during a process at constant pressure is equal to the change in enthalpy, or ΔH. The SI unit is joules. It is a state function. The basic equation: H = U + pV where U is internal energy, p is pressure, and V volume. The expression pV equates to thermodynamic work. Thus ΔH = ΔU + ΔpV, and at constant pressure (Δp = 0) and ΔH = ΔU + pΔV
Entropy: The measure of the amount of energy dispersed within a system so that the system can exist as a solid, liquid, or gas. Example. Vapourization involves a very large expansion and dispersal of molecules from the liquid. The total system energy does not change, but the way it is dispersed does change. Can also define entropy as the measure of all possible configurations of a system – i.e. how many ways can we arrange the components of the system?. In statistical mechanics, this is commonly stated as a tendency to disorder. Thus, an ordered system (e.g. a crystal) has fewer possible configurations than a disordered system (e.g. a gas). The 2nd Law of Thermodynamics states that a process will occur spontaneously if it increases the total entropy of that system. Basically, this means that the entropy of the universe is always increasing. It is usually given the symbol S – having SI units of J/Kmol. The concept is also applied to other fields of human behaviour and endeavour, such as social order. A paper by J.S. Martin et al., 2013 (Open Access) is quite a good explanation of the problems engaged in defining entropy.
Eogenetic: A stage of diagenesis that takes place between the latest deposition and burial beyond normal surface processes. The lower boundary is pretty fuzzy, because surface-derived groundwater can extend to variable depths depending on topographic potential. Originally defined by Choquette and Pray, 1970.
Epitaxial overgrowth: Cement overgrowths that are not in optical continuity with the substrate grain, and have a different mineral composition. Cf. syntaxial overgrowth.
Equilibrium constant: For a specific reaction, equilibrium constants are the ratio of product activities (or concentrations) divided by reactant activities; they can be determined experimentally (assuming a reaction is at equilibrium) or using thermodynamic considerations (where activities must be used). The general expression for a reaction involving ionic species in solution is: aA + bB ↔ cC + dD, where a, b, c, and d are the stoichiometric values for each ion (e.g. 2H+).
K = cC + dD/ aA + bB at equilibrium.
In a real aqueous solution, we can determine whether a reaction will proceed to the left or right: if cC + dD/ aA + bB is <K the reactants will convert to products (the reaction goes to the right. The opposite occurs if cC + dD/ aA + bB >K.
K is strongly dependent on temperature and pressure.
Euxinic conditions: Ocean waters that are depleted in dissolved oxygen (anoxic) and are sulphidic. The sulphide is primarily dissolved H2S. Euxinia can occur in highly stratified water bodies, such as lakes and enclosed seas where there may be an the anoxic layer occurs beneath shallower waters with varying amounts of dissolved oxygen. However, euxinia may also have occurred in larger oceanic water masses in the geological past.
Evaporative pumping: In arid regions, intense evaporation at the surface creates a hydraulic gradient in shallow subsurface aquifers, inducing lateral groundwater and/or seawater flow to replace lost fluid. Vertical capillary flow through the unsaturated zone (above the watertable) transfers these saline fluids from the aquifer to the surface.
Evaporite: Crystalline deposits formed by evaporation of marine and lacustrine brines. The most common products are gypsum, anhydrite, and halite, but also includes other sulphates, chlorides, and carbonates, including potassium salts like carnallite (KCl.MgCl₂·6H2O) and polyhalite (K₂Ca₂Mg(SO₄)₄·2H₂O). Precipitation commonly follows a sequence depending on the initial component concentrations and the changes in composition as evaporation proceeds and mineral phases remove ionic constituents from solution. For example precipitation of calcite will remove some Ca2+ that is now not available for gypsum (CaSO4) until evaporation further increases the Ca2+ activity.
Exothermic: A chemical reaction that releases energy, usually as heat. Cf endothermic. Common examples include many oxidation reactions, and addition of a strong acid to water.
Fascicular optic calcite: Void filling cement that consists of radially fibrous calcite clusters, where the crystal optic axes diverge towards the centre of the void. in concert with diverging crystals. Cf. Radiaxial fibrous cement.
Ferric (iron): Fe3+, or Iron III. is the common oxidized state of iron. It is the primary form of iron in limonite (FeO(OH)·nH2O) and hematite (Fe2O3). Magnetite (Fe2+ Fe3+2 O3 contains both iron II and iron 111. The oxidised state produces the red colouration in red beds and red shales.
Ferrous (iron): Fe2+, or Iron II. This is the common reduced state of iron in aqueous solution and common minerals like siderite (FeCO3), iron sulphate (FeSO42-), iron sulphide (FeS), and pyrite (FeS2). It combines with iron III in magnetite, and substitutes for calcium (Ca2+) in ferroan calcite, and for magnesium in ferroan dolomite. Iron II is largely responsible for the greenish hues of reduced shales.
First Law of Thermodynamics: In its simplest form, this states that energy cannot be created or destroyed, but it can be converted into heat and work. For example, at a macroscopic scale rain drops in a cloud have potential energy because of gravitational forces, some heat energy, and some kinetic energy derived from turbulence – most of this energy is converted to mechanical work as the drops fall; at a microscopic scale there may be heat loss. The total energy for a single rain-drop travelling to the surface will not change regardless of its travel path and longevity.
Fluid inclusions: Microscopic bubbles trapped in a crystal as it precipitates, contain samples of the fluid from which the minerals were originally derived. The fluid occurs as a single phase – liquid or vapour, or two phases with both liquid and gas. Most inclusions are less than 100µm (0.1mm) long. The fluids within may be fresh water or brines, or hydrocarbons. Samples are usually viewed in thin sections, gradually heated until the liquid and vapour homogenize into a single phase. The homogenisation temperature is generally considered to be the temperature at which the mineral crystal formed.
Framboid: Small, spherical aggregates of microscopic crystals, commonly measured in diameters of microns to 10s of microns. One of the more common occurrences is pyrite. They can form as primary precipitates in aqueous environments, or as diagenetic products. From the French framboise for strawberry.
Freezing point: The temperature at which there is a phase change from liquid to solid; also dependent on pressure. The standard pressure for the freezing point for water at 0o C is air pressure at sea level. At high elevations and lower atmospheric pressures, the freezing point of water is lower. The addition of a solute like salt (NaCl) will lower the freezing point – typical seawater freezes at -2o C.
Fugacity: A thermodynamic function used instead of effective partial pressures for reactions involving gases and gas mixtures and is a function of the chemical potential. It is usually expressed as a natural log – ln(f) = dμ/RT where μ is the chemical potential and R is the Gas Constant R = 8.314 J / mol·K. Thus, the fugacity of oxygen in air is the partial pressure exerted solely by oxygen.
Gamma ray: High energy, short wavelength particle emitted from the nucleus of an unstable isotope. It usually accompanies beta and alpha particle emissions. Gamma radiation is always emitted during nuclear fission in stars and explosive devices. It is one of the most dangerous forms of radioactive particle emission.
Gas constant: (also universal gas constant; molar gas constant) Given the symbol R, it is commonly expressed as R = PV/T where T is in Kelvin, V is molar volume, and P is pressure. R = 8.3144598(48) J/mol⋅K
Geopetal: Textures and fabrics that allow the interpretation of stratigraphic top, or ‘way-up’. This definition would include normal grain size grading in a turbidite. However, there is a tendency these days to restrict the meaning to structures where cements or sediments partially fill a void, such that the top of the fill represents a depositional or precipitation surface. Examples include fossils that have preserved chambers, the interstices between pillow lavas, and cavernous porosity in reef frameworks or caves.
Geothermal gradient: Temperature generally increases with depth in the crust; the gradient for a particular location is stated as the temperature increase per unit depth. The global average is 3o C/ 100 m although there can be large departures from these values in regions of geothermal and volcanic activity, or regions that have cooled significantly over geological time, such as old oceanic crust.
Gibb’s free energy: The energy given off or absorbed during a reversible reaction-process at constant temperature and pressure. It is defined as G = H – TS (H is enthalpy; S is entropy). Importantly, the change in G in a reaction will indicate the degree to which it spontaneously approaches equilibrium (ΔG is negative) or whether the reaction needs additional energy input (ΔG is positive). It is one of the more fundamental variables in thermodynamics.
Gibb’s Phase Rule: To solve problems of chemical equilibrium we need to know what variables are involved, those for which we have data and those we do not (unknowns), plus parameters like solubility constants and species activities. Solutions are found by solving simultaneous equations that express known and unknown variables. Gibbs Phase Rule is one way to formulate the problem. It is written as: F = C – P + 2. F is the degrees of freedom, which indicates the number of independent constraints needed to define the system in question (such as temperature, pressure, activity, concentration, electrical neutrality); C is the number of components, and P the number of phases. If F = 2 then two simultaneous equations are required to solve for the two unknown constraints.
Glauconite: A distinctive green to dark green-brown, K-Ca-Na sedimentary mica that usually forms as clay-like crystal masses, as cement fill in fossil chambers (foraminifera, gastropods, corals) or as replacement in fecal pellets. Strongly pleochroic in shades of green and brown although individual crystals are usually too small to identify and identification of pleochroism can be difficult. Monoclinic, biaxial (-).
Goldschmidt classification: The grouping of elements according to their place in the periodic table and their preferred mineral-forming phases. The four main groups are:
- Lithophile elements – those that bond readily with oxygen; tend to concentrate in the crust: Al, At, B, Ba, Be, Br, Ca, Cl, Cr, Cs, F, I, Hf, K, Li, Mg, Na, Nb, O, P, Rb, Sc, Si, Sr, Ta, Th, Ti, U, V, Y, Zr, W, plus the Lanthanides.
- Siderophile elements – iron-loving, mostly avoid oxygen, concentrated in the core and mantle: Au, Co, Fe, Ir, Mn, Mo, Ni, Os, Pd, Pt, Re, Rh, Ru.
- Chalcophile elements – bond with sulphur to form insoluble sulphides – low affinity for oxygen. The elements: Ag, As, Bi, Cd, Cu, Ga, Ge, Hg, In, Pb, Po, S, Sb, Se, Sn, Te, Tl, Zn
- Atmophile elements – H, C, N, noble gases: mostly form gases.
Greenhouse effect: The heating of an atmosphere when gas molecules absorb certain frequencies of solar infrared energy. On Earth this involves water vapour, carbon dioxide, methane, and to a lesser extent nitrous oxide. Molecular oxygen and nitrogen do not absorb infrared energy. Carbon dioxide and water vapour absorb energy at different frequencies. Note that the amount of water vapour in the atmosphere depends on temperature, unlike carbon dioxide.
Groundwater residence time: The time from recharge (usually at the surface) to discharge. Residence times are briefest in unconfined aquifers, ranging from days to years. In regional groundwater flow systems these times are measured in 105 to 106 years. Groundwater dating utilises trace compounds such as fluorocarbons, isotopes like ³H (tritium from atmospheric atomic device testing), and cosmogenic isotopes such as Carbon-14, and Beryllium-10.
Gypsum: CaSO4.H2O. Monoclinic, colourless, biaxial (+), good (010) cleavage bnut poor (100) and (111) cleavage, which means cleavage fragments do not generally show cubic patterns – cf. anhydrite. Slightly lower relief than anhydrite with RI from 1.52 – 1.53. A primary constituent of sedimentary evaporites in marine basins and terrestrial salt lakes-salars, along with other common minerals – anhydrite, halite, and sylvite.
Gypsum divide: The stage during evaporation of brines where gypsum precipitation determines the succession of minerals in waters subsequently depleted in Ca2+ and SO42-. It determines whether the brines evolve as SO4 rich – Ca poor, or SO4 poor.
Halides: Anions of the Chemical Periodic Table halogen group: Fluoride F‾, chloride Cl‾, bromide Br‾, Iodide I‾, and astatide At‾. Many inorganic halides are water-soluble; most organic halides are not.
Halite: NaCl. Isometric, colourless, usually cubic, and in thin section isotropic; refractive index is 1.54 (close to that of Canada balsam) hence low relief. Because of its solubility, thin sections need to be made using oil. In sedimentary rocks, can occur as a cement or as framework crystals – the latter are common in massive evaporite deposits. It has slightly higher relief than gypsum, and lower relief than anhydrite.
Halogens: The Periodic Table Group 17 elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astartine (At). Compounds from these elements are called halides. They are all corrosive, strong oxidizing agents. F and Cl are gases, Br is a brown liquid, I and At are solids.
Hexagonal crystal system: This system has 4 axes, 3 of which are perpendicular to c axis.
a1 = a2 = a3 ≠ c Angles between a1 = a2 = a3 = 120o
6-fold symmetry. Common crystal forms: Prisms, bipyramids. e.g., apatite, beryl. The Trigonal subsystem has one 3-fold axis or rotation. Three important examples are quartz, calcite and dolomite, commonly formed as bipyramids, rhombohedra, and scalenohedra.
Holomict: Lakes or seas in which there is mixing of surface and deeper waters. Bottom waters tend to be oxygenated Cf. Meromict.
Hydrolysis: Also called dissociation. The reversible reaction where H20 splits into a hydrogen ion and a hydroxyl ion, as in H20 = H+ + OH–. The equilibrium constant is written as:
Kw = (H+).(OH–)/( H20). The activity of H20 is usually taken as 1.0, so that Kw = (H+).(OH–). At 25ºC K= 10-14.0 . Where the concentration, or activity of (H+) > (OH–) is acidic, and (H+) < (OH–) is basic. This is the basis for the pH scale, calculated as the -log10 of the activities.
Hydrous minerals: (also called hydrates) Minerals that incorporate water into their crystal structure; their chemical formulae include either (H2O) or (OH). Common low temperature examples are calcite, gypsum, and halite (evaporites) and iron oxides like goethite (FeO(OH). Hydrous groups also occur in higher temperature-pressure minerals in metamorphic rocks (e.g., micas, amphiboles), and in mantle rocks (like olivine). Mineral hydration also occurs in subduction zones. The presence of hydrous minerals in the mantle helps to lower mineral melting points and promote partial melting.
Hygroscopic minerals: Minerals (and other materials) that absorb or adsorb water from their surroundings onto their crystal surfaces – commonly from air. One of the most common is halite – if enough water is absorbed the crystals can partly dissolve. (see deliquescent minerals).
Hypersaline: Having salinity greater than seawater (>35 parts/1000). Modern hypersaline environments are most common between the tropics but are found in such diverse places as the Antarctic dry valleys. Plant and animal life require specialized adaptations to survive these conditions. Prolonged hypersalinity may result in evaporite deposits in lakes and seas.
Induration: Refers to the degree of sedimentary rock hardening during compaction and chemical diagenesis, as temperatures, pressures, and fluid compositions evolve during burial.
Ion: An atom or molecule that has lost or gained electrons, giving it an electrical charge. Cations have positive charges from the loss of one or more electrons (e.g., Ca2+ is a calcium atom that has lost two electrons; anions have negative charges from gaining one or more electrons.
Ionic bonds: Formed between ion pairs that have positive and negative charges. Most ionic compounds are water-soluble – e.g., sodium chloride NaCl is formed from the electrical bonding of Na+ and Cl–. cf. covalent bonds.
Ionic strength: The sum total of ions in a solution (cations and anions). It is calculated as: IS = ½ ∑ ci zi2 where c is concentration (or activity) of species i, and z is the ion charge (the square of which is always positive).
Irreversible process: A rection or process that spontaneously goes in one direction but cannot do the reverse without doing additional mechanical or chemical work. For example, the expansion of gas, or the mixing of miscible liquids.
Isobar: A (contour) line connecting points of equal pressure in 2-dimensional space.
Isopachous cement: Cements that rim clast surfaces and are of approximately equal thickness throughout. Common types include aragonite and calcite as fibrous, bladed or drusy crystals, prismatic quartz, and clays like kaolinite and illite. They are commonly overlain by later pore-filling cements. Isopachous cementation implies fluid saturated pore spaces.
Isotherm: A (contour) line connecting points of equal temperature in 2- or 3-dimensional space. Cf isobar, isopach, isobath.
Isothermal process: A thermodynamic process that takes place at constant temperature. For example, if work is done to expand a gas, the temperature can only be kept constant if heat is transferred to the system. Cf. adiabatic process.
Isotope: For any chemical element, two or more species having the same atomic number (number of protons) but different mass number because of different numbers of neutrons. Isotopes of a particular element have the same number of electrons. Isotopes form part of nuclide decay series – some will be stable, others will decay to different isotopes. (e.g., 12C and 13C, 16O and 18O.
Kaolinite: A triclinic clay mineral with the general formula Al2Si2O5(OH)4, presented as flakes a few microns wide, or aggregates of flakes into mica-like books. A common weathering product of feldspars, a common diagenetic product as a cement or replacement mineral.
Karst: A landscape of gullies, canyons, and steep-sided pinnacles resulting from intense meteoric diagenesis (dissolution) of thick limestones. The relief on karst landforms ranges from 1-2 m to 100s of metres. The corresponding subterranean structures include sinkholes, caverns and underground streams.
Kelvin: The SI temperature scale that begins at absolute zero (−273.15 °C); zero degrees C is 273.15o K and 100o C is 373.15o K. Named after William Thomson (Lord Kelvin).
Kerogen: Kerogens are complex organic polymers that form during the breakdown of organic matter during the early stages of sediment burial. Three main types are identified depending on the O/C and H/C ratios of the polymer molecules: Type 1 is derived from algal organic matter, Type II from mainly marine micro-organisms, and Type III from plant material. Kerogen itself begins to break down at temperatures around 60o-80oC, as part of the organic diagenetic-maturation process. Identification of the kerogen types preserved in hydrocarbon deposits provides a good indication of the original organic matter.
Kinetic energy: The energy associated with a body or particle motion. It is stated as: EK = ½ mv2 where m is mass and v is velocity. It is a scalar quantity.
Laminar flow: Flow that is non-turbulent and can be expressed graphically by a series of parallel flow lines. Laminar flow is important at grain or other solid surface boundaries – the boundary layer and as such is an important consideration in diagenetic reactions involving mass transport of dissolved solutes. It is also important in larger-scale flow systems such as stream flow or turbidity currents. The distinction between laminar and turbulent flow is commonly described by the dimensionless Reynolds Number.
Le Chatelier’s Principle: When applied to chemical reactions, the principle established by Henry Le Chatelier in 1884, states that if conditions for the reaction change (temperature, pressure, concentrations) then the system will attempt to re-establish a new equilibrium. In other words, the system will adjust to compensate. For example, if the concentration of a reactant is increased the equilibria will move to the right (creating more product) until a new equilibrium condition is established. If a reactant concentration is decreased, then the reverse occurs and some of the product will dissolve or dissociate to compensate.
Limonite: Fe(OH).nH2O. An amorphous, soft, yellow-orange to red-brown hydrated iron oxide that usually occurs as the weathering product of iron-bearing minerals, as cements in some coarse-grained sediments, and ferricretes. It is commonly an indicator of ancient water tables in coarse-grained aquifers where it forms crusts and nodules. It is also responsible for the iron staining in Liesegang rings. Tends to be opaque in thin section. Commonly mixed with clays.
Lithification: The combination of compaction and cementation that produces hard, hammer-ringing rock from loose, uncompacted sediment. Lithification depends on a complex association of physical and chemical processes. Cementation can occur at very shallow depths in the case of carbonates, or at different stages of burial depending on temperature, and rock – fluid chemistry. Compaction begins soon after deposition and continues at depth.
Lithophile elements: One of the Goldschmidt classification groups of elements that readily bond with oxygen. This means they tend to be concentrated in Earth’s crust and probably the crusts of other rocky planets – common examples are Na, Ca, Mg, Si, Al, K. as well as some of the transition metal elements like Fe, Mn. cf. siderophiles.
Lysocline: The ocean water depth where the dissolution of calcite is first observed in sediment. Its identification requires detailed observation of dissolution textures and is somewhat subjective. It lies above the calcite and aragonite compensation depths; the lysocline should, theoretically, be close to the saturation levels for both minerals.
Magnesium calcite: Also called magnesian calcite. In the calcite crystal lattice, magnesium can occupy the position of calcium, up to about 20 mole percent. Two varieties predominate in carbonate sediments and limestones: Low magnesium calcites (LMC) with <4 mole % Mg), and high magnesium calcites (HMC) with 11-19 mole % Mg). HMC commonly recrystallize to LMC during burial diagenesis.
Mass: A measure of the amount of matter in an object. The SI unit is the kilogram (kg). The mass of an object is not dependent on the local force of gravity (g) – mass does not change even if the value of g changes. For example, a 1 Kg mass on Earth weighs 9.8 N; on the Moon the same mass weighs 1.62 N (Lunar value of g is 1.62 m.s-2), and on Jupiter it weighs 24.79 N where g = 24.79 m.s-2. Mass is not the same measure as weight.
Mass fraction: The ratio of the amount of a component (atoms, molecules, ions) to the total mass in a mixture.
Mass number: The total number of protons plus neutrons in an element. Cf. Atomic number.
Maturation: The process where organic compounds in sedimentary rocks are converted to hydrocarbons and byproducts such as organic acids. The term is normally used for hydrocarbon source rocks (e.g. black shales with high total organic carbon – TOC). Biological components from dead critters begin to convert at about 60oC, and more rapidly above 80oC. The conversion temperature window – the oil and gas window – is 80o to 120oC. Temperatures at the high end of this scale tend to produce light hydrocarbons and gas. Organic maturation is accompanied by pH buffering and various inorganic diagenetic reactions, particularly with clays, carbonates, and quartz.
Mechanical dispersion: In geofluids, this occurs when solute molecules are carried from the source by local eddies around grains or through fractures; this kind of tortuosity takes place at a scale much smaller than the en masse advective flow. Cf. Molecular diffusion.
Melting point: The temperature at which the solid and liquid phase are in equilibrium. The point at which a solid becomes a liquid (a phase change). The freezing point has the same numerical value.
Meniscus: The contact between a liquid and solid boundary where the surface becomes concave upward because of surface tension forces.
Meniscus cement: Cements that accumulate at clast contacts in vadose, or unsaturated conditions, where precipitation (commonly calcite or aragonite) takes place in the fluid meniscus. The meniscus itself is caused by surface tension forces at the boundaries of grains in contact.
Meromict: A stratified lake or enclosed sea where the layers do not mix. Bottom water layers may become anoxic as dissolved oxygen is used up by organisms. In saline waters it applies to salt crystals that precipitate within saturated layers and then sink to the bottom.
Mesogenetic: A stage of diagenesis that takes place at burial depths below normal surface processes, including surface topography-driven groundwater flow. Originally defined by Choquette and Pray, 1970.
Meteoric diagenesis (carbonates): Diagenesis of limestone under fresh-water conditions, both in the vadose (unsaturated) zone, and below the watertable. It is largely controlled by the degree of fresh- water seepage and groundwater flow. Vadose zone diagenesis is dominated by dissolution that, if prolonged, produces caverns, sinkholes (dolines), subterranean streams, and spectacular karst landforms. Dissolved calcium carbonate may reprecipitate as cement and fracture-fill in the saturated zone, and as stalactites-stalagmites in caves.
Micrite: A contraction of microcrystalline carbonate mud, refers to aragonite or high-magnesium calcite crystals less than 4 microns, that form as cements (usually isopachous cements), or the product of substrate alteration by boring algae, fungi, and larger critters like boring Clionid sponges – a process call micritization. Micrite is highly reactive and susceptible to recrystallization during meteoric and burial diagenesis because the original mineralogy is metastable, and because of the high surface area afforded by the micron-sized crystals.
Molality: A unit of concentration expressed as the number of moles of solute per kilogram of solvent.
Molarity: A unit of concentration expressed as the number of moles of solute per volume of solution measured in litres.
Mole: The standard SI unit for the amount of a substance that is equivalent to 6.022 x 1023 atoms (Avogadro’s number), measured as the number of atoms in 12 grams of C-12. Mole is abbreviated from the word molecule.
Mole fraction: An alternative unit of concentration expressed as the ratio of the number of moles of a component in a mixture to the total number of moles in the mixture. For example, if 1 mole of NaCl is dissolved in 1 liter of water (55.55 moles), the mole fraction of salt is 0.018. The molarity is 1 mol, and the molality is also 1.
Molecular diffusion: When a solute gradually mixes with solvent molecules; in geofluids this is primarily water. The process does not involve the physical flow of water, but depends on solvent-solute properties such as polarity and charge, and vibration energies. Cf. Mechanical dispersion.
Monoclinic crystal system: a ≠ b ≠ c α = γ = 90o, β ≠ 90o
2-fold symmetry.Common crystal forms: Prisms, pinacoids (flattened prisms).e.g., orthoclase, diopside, sphene, staurolite, most amphiboles.
Monocrystalline quartz: Applies to grains of quartz that consist of a single crystal. It is used in petrographic descriptions of arenite and coarser lithologies to distinguish this variety of quartz from polycrystalline quartz. See also strained quartz, volcanic quartz.
Neomorphism: Defined by R. Folk in 1965 as the transformation between one mineral and itself or a polymorph. In other words, neomorphism is a product of recrystallisation where the bulk composition does not change, only the size and/or shape of crystals. It is common in carbonate lithologies and involves recrystallisation of both framework clasts and cements. As such it tends to cross-cut pre-existing textures and fabrics; relict textures may be preserved. Aggrading neomorphism is common in micrites where crystals increase in size in a more-or-less radial fashion.
Nitrate: A water-soluble univalent anion of nitrogen, NO3–, common in fresh waters, particularly those subject to agriculture runoff, but much less common in seawater. Sodium and potassium nitrates are common additives in fertilizers. The conjugate acid is nitric acid (HNO3). Also used as a commercial food preservative. Nitrate minerals like sodium nitre are rare geologically, mainly because of their high solubility.
Nitrogen cycle: The natural transfer of nitrogen and nitrogen compounds from air to soils, vegetation, water and back to the atmosphere. The natural cycle is complicated by human interventions via fertilizers (nitrates) and industrial nitrogen oxides that saturate soils and leach into shallow groundwater and surface waters. Most of the natural nitrogen fixing is done by microbes.
Nitrogen fixing: This is an important process for plant uptake of nitrogen. Plants do not get their fill of nitrogen from the air, but from soil and plant microbes (fungi, bacteria) that convert molecular nitrogen in air (N2) to water soluble compounds, principally nitrates (NO3– ). Plants utilize this soluble form, taking it up via their roots.
Oncoids: Oncoids are rounded, spherical to oblate, concentrically laminar growths of algae around a nucleus (shells, mud intraclasts, broken lumps of algal crust). They grow at the sediment surface in supratidal (including sabkhas), intertidal and shallow subtidal environments that are agitated by currents and waves, and perhaps frequented by storm surges. Oncoid laminae grow asymmetrically while the oncoid is at rest, and discordantly when growth has been interrupted by clast jostling and rolling.
Oncolite: The rock name for limestone consisting of oncoids.
Ooids: Ooids are spherical to subspherical grains, typically 0.5mm diameter, characterized by concentrically layered, micron-sized calcite or aragonite crystals. They are common constituents of carbonate platforms and ramps where they form extensive bars and sandwaves. There is usually a nucleus of skeletal debris or pelloids. In saline lakes the ooids may consist of radial aragonite. Ancient ooids also show radial crystal fabrics. They precipitate from seawater, but there is mounting evidence that this is mediated by microbes. They are also known from Precambrian iron formations.
Oolite: A limestone made up predominantly of ooids. It is a rock name. There will usually be evidence for bedload transport of ooids, such as crossbeds and scours, or preserved ripple forms.
Oil generation window: The temperature range 80° – 120°C where hydrocarbon maturation to liquid oil from sedimentary organic carbon, is most rapid and most productive. At an average geothermal gradient of 30°C/km, the top of the window occurs at depths of about 3 km. Organic matter subjected to temperatures >120°C is prone to gas formation.
Oil migration: Hydrocarbon production in deeply buried sediments, begins in organic-rich sediment, such as oil shale. Once formed (by a series of complex chemical reactions), the oil (and gas) migrate from the shale or mudstone to more porous and permeable rocks such as sandstones and limestones. Migration is driven buoyancy forces and the flow of deep subsurface groundwater. Migration will continue until the oil is trapped (resulting in an oil field). Oil and gas that isn’t trapped will eventually find its way to the surface or sea floor and escape.
Oil seep: Oil, sometimes accompanied by gases like methane, that leak to the surface via fractures or faults, driven of buoyancy forces, or as a part of spring flow. The hydrocarbons may be sourced from oil-prone porous rock, or from actual subsurface oil pools.
Organic acid: Like other acids they donate protons during reaction. The most common forms are carboxylic acids that have the general formula R-COOH where R is an alkyl group, the simplest being -CH3 (methyl) in acetic acid (CH3-COOH). They are important contributors to carbonate diagenesis during maturation of organic matter; the acids can also buffer pH in diagenetic flow systems.
Orthorhombic crystal system: a ≠ b ≠ c α = β = γ = 90o
2-fold symmetry. Common crystal forms: Prisms, bipyramids.e.g., olivine, cordierite, hypersthene
Oxidation: The process where an atom provides electrons to another atom of a different element; and oxidized element has lost electrons. Oxidation always occurs with reduction (REDOX reactions). An oxidized element (atom) is capable of gaining electrons, in which case it becomes reduced; the initial oxidized element is referred to as a reducing agent. Thus Fe2+ is more reduced than Fe3+ ; in the mineral pyrite FeS2 iron is in the 2+ state and sulphur -1 state.
Ozone: When oxygen molecules (O2) in the stratosphere are bombarded with high energy ultraviolet light (UV) the molecule splits into two oxygen atoms. Each of these atoms in turn reacts with O2 to produce ozone, or O3. Ozone is responsible for absorbing some of the harmful UV radiation that would otherwise reach the surface of the Earth.
Paleothermometer: Geological, paleontological and chemical tools used to determine the temperature conditions and thermal history of ancient environments, and more deep-seated processes associated with sedimentary basins, igneous and metamorphic events. They are components of rocks such as minerals, isotopes, fossils, and fluids that provide us with either a direct measure or proxies of paleotemperatures. Common examples include vitrinite reflectance of coals, fossil colour, radiogenic blocking temperatures, stable isotopes of oxygen and carbon, fission tracks, and fluid inclusions.
Paragenetic sequence: In sedimentary petrology, the sequence of mineral components precipitated (and dissolved) during diagenesis. Sequential changes in mineral composition and/or crystallographic form reflect evolving fluid compositions, fluid flow, burial temperatures, and compaction. It is analogous to cement stratigraphy.
Parent and Daughter nuclides: For radioactive nuclides, a parent nuclide is the starting point for decay of a particular element (unstable) and the product(s) are the daughter nuclides. Daughter nuclides may also be unstable and decay further, the products sometimes called grand-daughter nuclides.
Path function: In thermodynamics and classical mechanics, the property or variable that depends on the path taken to get from one state to another state, and not just on the initial and final states (as in state functions). A good example is Work – for example a rain drop that falls vertically to Earth requires less mechanical work that a drop that move sideways on its trajectory – Work is dependent on the path taken. Heat is another common example.
Pelloids: Any spheroidal, sand sized grain that is an aggregate of micro- to cryptocrystalline carbonate. There is little or no internal structure. If they are known to be fecal then they are called pellets. Otherwise, the term pelloid should be used.
Pendant cement: Stalactite-like cements that accumulate on the low point of grains during gravity drainage of interstitial fluid. They are common in carbonates subjected to vadose zone diagenesis.
Periodic table: The earliest form of the modern Table was introduced by Dmitri Mendeleev in 1869, who arranged the known elements according to atomic weight. He demonstrated that there were gaps in the table structure, that later were filled as additional elements were discovered. The modern Table contains element rows based on atomic number, and columns that correspond to element groups – the more metallic groups are on the left side of the Table (Group 1 is alkali metals, Group 2 alkali earth metals, and so on. Groups to the right are increasingly non-metallic. The far-right column contains the noble gasses of Group 18. Elements with atomic numbers 95 to 118 are synthetic. Modern tables usually include information on electron configuration, isotopes, density, ionization energies, availability, and their origin in the context of stellar processes.
pH: Literally the ‘potential of hydrogen’, is a measure of the acidity or alkalinity of an aqueous solution. It is expressed as:
pH = -Log10 (aH+) where aH+ is the activity of H+ in solution.
This means that high concentrations of H+ have low pH values. The pH range is 0 to 14; a neutral solution has pH = 7. An acidic solution has a pH <7.0; an alkaline solution >7.0. Pure water at 25oC has a pH of 7; rain a pH of 5.0 to 5.5 (i.e. slightly acidic because of dissolved CO2), and seawater 7.5 to 8.1. The variations are partly dependent on temperature and its influence on the carbonate equilibria.
pH buffering: Carbonate equilibria do not operate in isolation. If the amount of dissolved CO2(aq) is increased this does not mean that the amount of H+(aq) will increase by the same amount because some of the CO2 forms H2CO3 (aq), some HCO3– (aq), and some CO32- (aq), such that the amount of H+ added is small. In other words, the cascade of equilibria acts to buffer the system against large changes in pH.
Phase (thermodynamics): The physical state of a substance – gas, liquid, or solid. Plasmas are considered by some as the fourth phase. Phases are also defined by the potential for mixing at the molecular level, and by the interfaces between phases. For example, a system containing crystalline calcite and aragonite would have two phases. In aqueous systems two liquids will always mix and hence there is only one phase; this also applies to different gasses. However, a system with aqueous and hydrocarbon liquids would have two phases.
Phase diagram: The graphical representation of different states for a compound, as solid, liquid, or gas. The phase diagram for water is plotted as pressure against temperature; the triple point where all three phases coexist is at 0.01oC and 608 pascals (0.006 atmospheres). For carbon dioxide the diagram also shows gas, solid and liquid phases, plus a supercritical liquid phase.
Photosynthesis: A process that converts sunlight energy to chemical energy in plants, cyanobacteria, and algae. One of the chemical products is molecular oxygen(O2), that in plants is formed from carbon dioxide reacting with water in plant cells to produce sugars and oxygen. It is generally understood that most of Earth’s free oxygen was produced during the Precambrian by cyanobacterial stromatolites.
Photic zone: The uppermost layer of the oceans and lakes where light penetrates; the base of the zone is at about 1% of incident sunlight. On average it is about 200 m deep. It is the layer where more than 95% of photosynthesis by marine organisms takes place.
Piper diagram: A matrix of three triangular plots that map the chemical compositions of water. It is based on normalized percentages of major cations (Calcium, magnesium, potassium, and sodium), and carbonate-bicarbonate, sulphate, and chloride anions. It is useful for tracking the source of groundwater flows in aquifers derived from different rock types, and the evolution of chemical speciation.
Pisoid: Concentrically layered ovoid to markedly elongate carbonate bodies that superficially resemble oncoids. However, unlike oncoids and ooids, pisoids form in meteoric vadose conditions, commonly associated with calcretes (paleosols). Common textures include: close-fitted and intergrown pisoids (hence their shapes are irregularly elongate), evidence of multiple stages of precipitation and dissolution (commonly as cross-cutting fabrics), and gravitationally-induced pendant cements.
Pisolite: A limestone made up predominantly of pisoids. It is a rock name. Commonly form in vadose zone soils or caliches.
pK: The negative log of an equilibrium constant (K).
Potential energy: This is the energy available for a system to do work. From a mechanical perspective, a drop of water at rest has the potential energy equivalent to the product of the drop mass, it’s height above some datum, and the gravitational constant – written as:
PE = m.h.g
If the drop falls, some of this potential energy is converted into kinetic energy (and possibly some thermal energy) and the change in PE is ΔPE = m.g.(h2 – h1). The choice of a datum is arbitrary – for example the centre of the Earth, sea level, or some other local surface.
Ppb: Parts per billion
Ppm: The abbreviation for parts per million. For water this equates to 1mg/Litre.
Ppt: The abbreviation for parts per thousand. Also written as ‰.
Pressure solution: The dissolution of rock components (framework clasts and cements) as a result of differential compressive stress. Common products of pressure solution are stylolites. Conditions required for dissolution to take place are:
- Differential compressive stresses develop at intergranular contacts,
- Interstitial fluids must be undersaturated with respect to the mineral phase under stress,
- Dissolved components are transported from the grain contacts to regions of lower compressive stress; this requires efficient fluid movement, and
- The solute reprecipitates some distance from its point of origin.
Poikilotopic cement: Growth of large crystals (commonly calcite, dolomite) that enclose several/many framework grains. In thin section, the enclosing crystals have uniform extinction (under crossed nicols) whereas the individual framework grains will usually have disparate extinctions.
Polycrystalline quartz: Used to description of sand-size and larger quartz grains (usually in thin sections) that consist of two or more, usually more, subcrystals. Subcrystal boundaries are generally irregular – even embayed. Under crossed polars each subcrystal will move into extinction at different stages of thin section rotation. The mechanical breakdown of polycrystalline grains produces very fine sand to silt-sized particles. This category of quartz is common in arenites; the most common source is metamorphic rocks.
Pore throat: The narrow passages between grains in contact, that connect the larger intergranular pores. Pore throat sizes are variable, depending in part on the packing arrangement of grains and grain shapes, and range from submillimetre to a few microns. Their size and distribution are a primary control on the characteristics of fluid flow. Pore throats can be blocked and their efficacy reduced by cements, particularly clays.
Protodolomite: The term was originally applied by Graaf and Goldsmith (1956) to imperfectly ordered dolomite with excess calcium, later modified to include ordered lattices. Whether this is a useful definition is still debated. It commonly refers to geologically Recent, disordered dolomites, many of which are found in sabkha-like environments and saline lagoons. Production of protodolomite and dolomite in low, Earth-surface temperature lab experiments is difficult – common products in these experiments are high magnesium calcite brucite (Mg(OH)2), and magnesite (MgCO3).
Proton: A subatomic particle having positive electric charge, that with neutrons, forms the bulk of an atom nucleus. It is usually given the symbol H⁺.
Psi: Pounds per square inch. A non-SI unit of pressure. The standard is the air pressure of 14.696 psi at mean sea level, or 1 atmosphere, or 101.325 kPa.
Pyrite: FeS2. A common accessory, opaque mineral in many metamorphic and igneous rocks, in hydrothermally altered sedimentary rocks, as an authigenic mineral in anoxic sediments (e.g., black shales), and as a diagenetic product when reduced iron is available. (Fe is in a reduced state). Forms isometric crystals with cubic habit, singly or in clusters. Oxidizes to limonite (FeO(OH) · nH2O) and goethite (FeO(OH)) and hence is less common as a heavy mineral in sedimentary rocks.
Quartz: One of most common detrital constituents of sedimentary rocks, and second only to feldspar to all rocks in general. Its chemical formula is SiO2. Crystallographic form is hexagonal rhombohedral. It is uniaxial positive and non-pleochroic. Strained quartz will show undulatory extinction. It occurs in felsic igneous and metamorphic rocks, hydrothermal deposits, fracture fills, and as diagenetic products (e.g. cements). It forms chert and chalcedony in its micro- and cryptocrystalline form.
Radiaxial fibrous cement: Radiaxial fibrous calcite and its companion fascicular optic calcite are common cavity-filling cements in ancient limestones. Both consist of radially fibrous calcite clusters but differ in their crystallographic orientation. Cleavage and twin planes in radiaxial crystals are concave toward the pore interior, in concert with optic axes that converge toward pore spaces (i.e. opposite the divergence in the actual crystals). In some cases, the fabric may represent calcite-dolomite replacement of initially fibrous aragonite. In others the fabric may precipitate directly.
Random errors: Errors of measurement usually with an unknown cause; they are unpredictable, unlike systematic errors. For example, errors in temperature (from expected values) caused by fluctuations in fluid turbulence, unexpected electrical or acoustic noise. If the sources of error can be identified they can sometimes be filtered out – for example acoustic noise caused by traffic rumble.
Reaction kinetics: See Chemical kinetics.
Recrystallisation: In sedimentary rocks this involves the transformation or replacement of a mineral with itself, and usually entails changes in crystal size and shape (but not bulk composition): as in micritic calcite to sparry calcite, or aragonite to its polymorph calcite. The term was originally coined for the process of annealing in metals, which is a dry process. Recrystallisation in sedimentary rocks is always a wet process that involves dissolution of a mineral at grain boundaries, followed by precipitation. It tends to cross-cut original textures, destroying them in the process.
REDOX: Reactions in which oxidizing and reducing agents combine; thus one atom is oxidized and the other reduced simultaneously. For example, in the sour, toxic gas hydrogen sulphide (H2S), 2 H atoms lose an electron each to the sulphur atom; 2H+ S2-.
Redox reaction: See Reduction and Oxidation.
Reduction: When an atom gains electrons it becomes reduced. It has electrons to spare and can donate them to the atom of another element that has a deficit of electrons (i.e. it is oxidized). A reduced element that donates electrons is a reducing agent. Cf. Oxidation, REDOX.
Relative humidity: In air at any given temperature there is a water vapour saturation limit. Relative humidity is the ratio of the actual vapour pressure to the saturation pressure at that temperature.
Resistivity: (ρ) A property of all materials, it is a measure of the ability to conduct or resist and electrical current. It is defined as the electric field strength divided by the current density. Highly resistive materials are good conductors. Resistivity measurements are fundamental to subsurface exploration (hydrocarbons, minerals), defining deep Earth structures, and subsurface mapping. Borehole logging commonly includes resistivity tools.
Saline lake brines: Unlike seawater, terrestrial brines have widely variable compositions, depending on local soil and bedrock compositions, groundwater chemistry, and the degree of evaporitic drawdown. Typical brines contain Na+, Ca2+, Mg2+, Cl–, SO42-, HCO3–, CO32-, and SiO2, but concentrations are highly variable. pH ranges from highly alkaline to highly acidic. Evaporation pathways produce a succession of different minerals. See also calcite-gypsum divides.
Salt: the general name for a solid compound formed by the reaction between an acid and a base.
Saturation: Saturation (Ω) is the ratio of the measured ion activity (or concentration) product and the standard solubility product (Ksp) for a mineral. If Ω >1 then the solution is supersaturated with respect to the mineral; if Ω <1 then it is undersaturated and the mineral will dissolve. If Ω = 1 then the mineral is at equilibrium with the solution.
Saturation depth: In ocean chemistry this boundary identifies when seawater becomes unsaturated with respect to calcite (or aragonite). The saturation depth is determined by comparing the measured solubility product of either the activity or concentrations of Ca2+ and CO32- in seawater samples, with the thermodynamic equilibrium solubility product at the same temperature and water pressure.
Secondary porosity: Porosity that is created during burial diagenesis by the dissolution of chemically reactive grains such as carbonates and feldspars. Secondary porosity can enhance the overall porosity of a rock, particularly if primary intergranular pore volumes have been occluded by cements. Secondary pores may be larger than those formed during deposition, where entire grains are dissolved. Partial dissolution along twin or cleavage planes in minerals like feldspar, will result in irregular grain boundaries.
Second Law of Thermodynamics: One of the unifying concepts that links all changes in physical and chemical systems. It states that any process will take place spontaneously if it increases the total entropy of the universe. Entropy is sometimes likened to disorder – the expansion of a gas increases the degree of disorder, and therefore entropy. The word ‘universe’ is important here because some processes at a local scale decrease disorder, or entropy, such as precipitation from aqueous solution to some crystal form – in this case mass is more ordered – also referred to as configurational entropy. However, even in this example, thermal entropy increases. Entropy is a state function – its value is independent of the path the system changes take.
Sequestration: Storage of solid, liquid or gas so that it cannot disperse, or escape. Of recent concern is sequestration of carbon in various forms, particularly CO2 and methane. Natural sequestration occurs on rocks (coal, limestones), soils, and permafrost. Artificial sequestration of supercooled CO2 in certain rock formations (such as depleted oil fields) is considered as one means of controlling CO2 emissions.
Sericite: A flaky white mica and common alteration product of feldspar. In thin section it usually presents as fine ragged crystals (rather than the more uniform muscovite flakes), concentrated along feldspar cleavage planes, or distributed across the entire crystal. It has high birefringence and appears to sparkle against the dull background of altered grains and matrix.
SI units: The international system of units, formalized in 1960, contains 7 base units: weight in kilograms (kg), length in metres (m), time in seconds (s), electric current in amperes (A), temperatures in Kelvin (K), amount of substance in moles (mol), and light intensity in candela (cd). Units such as pressure are derivatives of the base units – in this case expressed as pascals (Pa) which is equal to one newton per square metre (N/m2 or kg m-1s-2).
Siderite: (Fe,Mg)CO3 – FeCO3. An iron carbonate commonly found in mudrocks, as concretions in paludal deposits, and as ooids. In hues of grey to orange brown. Also as a hydrothermal alteration mineral. Hexagonal, uniaxial (-), very high relief and birefringence. Alters to limonite and goethite.
Siderophile elements: Literally iron-loving elements, they include the high density transition metals that bond with iron in solid and molten states. They can also bond with sulphur and carbon. As such they tend to concentrate in Earth’s core and to a lesser extent the mantle. They re rare in the crust. Most, except for Fe and Mn have a low affinity for oxygen. The list includes Ag, As, Bi, Cd, Cu, Ga, Ge, Hg, In, Pb, Po, S, Sb, Se, Sn, Te, Tl, Zn – Sulphur is also a volatile element and at Earth’s surface combines with oxygen to form sulphate anions.
Silica: The compound SiO2, named silicum by Humphrey Davy in 1808, from the Latin for flint – silex. The mineral quartz has the same chemical composition and formula. Silica is the basic compound for most minerals in Earth’s crust and mantle, usually referred to as silicates.
Silicate: The group of minerals that contains the tetrahedral-shaped anion SiO44- where Si has an oxidation state of 4+. It is estimated that 90% of the crust is made up of silicate minerals. Common mineral groups include feldspars, ferro-magnesians and clays. Silicates are classified according to the number of tetrahedra connected by shared oxygen atoms; the molecular groups are organized to form simple 3-dimensional structures like quartz, or when metal cations are bonded to single tetrahedra to form minerals like olivine, sheets (e.g., micas), single chains (e.g., pyroxenes) and double chains (amphiboles), and rings (e.g., beryl).
Silicon: The non-metallic element Si, atomic number 14, discovered by Swedish chemist Jöns Berzelius in 1824, but the name silicon was applied by Scottish chemist Thomas Thomson in 1831. The name is derived from Latin silex meaning flint.
Solid solution series: Minerals that share the same basic chemical formula but have different proportions of key elements in their crystal lattice such that crystal form may vary. In sedimentary rocks the most important examples are the alkali (K-Na end-members) and plagioclase (Na-Ca end-members) feldspar groups. Olivine also forms a series with fayalite and forsterite end-members. A mineral’s position in a series reflects the composition and temperature of, for example, the original igneous melts (in the case of feldspar and olivine.
Solubility product: Solubility product expresses whether a mineral will dissolve or precipitate in aqueous solutions, at specified temperatures and pressures. For example, aragonite in seawater, the reaction is CaCO3(solid) ↔ Ca2+(aq) + CO32-(aq). At equilibrium the solubility product is
Ksp = (aCa2+).(a CO32-) / (a CaCO3 solid)
The activity of the solid calcite is 1, such that the constant at equilibrium becomes:
Ksp = (aCa2+).(a CO32-)
(aCa2+).(a CO32-) is also called the activity product. In real solutions, if (aCa2+).(a CO32-) is >Ksp, then aragonite will precipitate; if <Ksp it will dissolve. See also saturation.
Solute: A chemical compound that has dissolved in a solvent. In geofluids, the solvent is primarily water; common solutes are various chlorides, sulphates, hydroxides, nitrates and phosphates. In all these compounds, the solute will consist of cations and an anions surrounded by water molecules.
Solute transport: The movement or flow of dissolved mass in a fluid, usually water. The primary mechanisms of transport are advective flow and diffusion. Transport is usually accompanied by chemical reactions.
Solvent: A liquid (usually) capable of dissolving and maintaining solutions of solid compounds. Water is the most prominent geofluid solvent. Organic solvents are important for industrial processes.
Stalactite: Tubes, straws. and threads of calcite that hang from the ceiling in the drip zone of caves. Groundwater, initially undersaturated with respect to calcite can, with sufficient transfer of atmospheric CO2, become supersaturated, promoting precipitation. Pillars or columns form when stalactites meet stalagmites, their cave-floor counterpart. They are a type of speleothem, a group of cave precipitation structures that includes cave wall linings (drapery), flowstone, and cave pearls. Stalactites and stalagmites can also form from dripping lava.
Stalagmite: Commonly conical shaped mounds of calcite that grow from cave floors as a result of the steady drip of seepage groundwater. They are the cousin of stalactites.
State function: In thermodynamics and classical mechanics, a property or variable that depends only on the initial and final states of some process and not the path taken or history to get from one state to another. For example, if a physical or chemical process involves a change in volume, it doesn’t matter what path that volume change takes, only the start and end volumes. Volume is a state function. Again, a chemical reaction (e.g. precipitation of calcite) may take different paths from initial final states, but the total enthalpy and Gibbs Free Energy will be the same regardless of the reaction path. Pressure, temperature, density, mass, entropy, enthalpy, and Gibbs Free energy are also state functions. Cf. Path functions.
Stoichiometric coefficient: Quantifies the number of atoms, ion, molecules for each reactant and product in equilibria. The standard notation is: aA + bB D cC + dD where a,b,c, and d are the coefficients for components A,B,C, and D.
Stoichiometry: An analysis of the relationship between the products and reactants in a chemical reaction, written in the form of an equation. It expresses the number of elements, ions, or compounds required to balance the equation. For example the precipitation of dolomite from solution Ca2+aq + Mg2+aq + 2(CO32-)aq D CaMg(CO3)2 balances cations and anions with the final product. In this case the stoichiometric coefficient of CO32- is 2, and that of Ca2+ and Mg2+ is one.
STP: Standard Temperature and Pressure. It was defined by the International Union of Pure and Applied Chemistry as 273.15 K (0 °C) and an absolute pressure of 100 kPa (1 bar). STP is used for base values for comparisons and corrections of temperature and pressure measurements. STP is commonly used for standardizing thermodynamic variables.
Strong acid: Strong acid: Strong acids dissociate completely – they donate all their H+ to form H3O+. Common examples include hydrochloric acid (HCl) and sulphuric acid (H2SO4).
Structure grumeleuse: A term introduced by Lucien Cayeux in 1935, refers to clotted limestone textures where isolated, diffuse patches of micrite are surrounded by coarser neomorphic spar; the overall texture appears clotted. At times it can be difficult to distinguish between this recrystallisation texture and primary peloidal limestones.
Stylolite: Saw-tooth like, discordant seams that signify pressure solution of rock components (framework clasts and cements). They are most common in carbonates but can form in siliciclastic rocks. They represent differential compressive stresses at grain-to-grain contacts, the dissolution and mass transfer of carbonate by diffusion and fluid flow. Stylolites commonly parallel bedding (from normal compressive stress) but also form oblique to bedding.
Sublimation: The transformation of a solid phase to a gas without an intermediate liquid phase. Solid CO2 (dry ice) is a well known example that sublimates at temperatures below its freezing point at -78o C.
Sulphate (sulfate): A compound anion, SO42-, one of the most common in aqueous environments, particularly seawater. It forms from the oxidation of sulphur (S) and sulphur dioxide (SO2 gas) commonly emitted as volcanic gas, or from the oxidation product of metal sulphides like pyrite. All metal sulphates are soluble. It is the soluble salt of sulphuric acid (H2SO4). The most common geological minerals are gypsum and anhydrite (CaSO4), and less common barite (BaSO4). Sulphate – sulfate was named by French chemist Louis-Bernard Guyton de Morveau in 1787, from the Latin sulfur and sulphatus. The word sulphat is found in the English version of Antoine Lavoisier’s Traité élémentaire de chimie (1789) by translator Robert Kerr in 1790. In Lavoisier’s original text he makes frequent reference to l’acide sulfurique (sulfuric acid), and in Chapter 17 he defines the salts of various acids: “Ainsi nous avons désigné tous les sels qui ont l’acide sulfurique pour acide, par le nom de sulfates; tous ceux qui ont l’acide phosphorique pour acide, par le nom de phosphates, & ainsi des autres.” that translates – “Thus we have designated all the salts which have sulfuric acid for acid, by the name of 185 sulfates; all those which have phosphoric acid for acid, by the name of phosphates, and so on with the others.”
Supersaturation: A solution in which the concentration of a dissolved compound exceeds saturation, such that the ratio of the measured ion activity (or concentration) product and the standard solubility product (Ksp) is >1.
Syntaxial overgrowths: Crystals that have grown on, and are in optical continuity with a substrate (of the same composition) are referred to as syntaxial overgrowths or cements; i.e. they share the same optic axis (the term epitaxial is also used in this sense by some, although the International Mineralogical Association insists that in epitaxy the overgrowing phase is mineralogically different to the substrate). Well known examples involve quartz overgrowth cements in quartz arenites; in many cases the original grain outline is distinguished by a rim of inclusions. In limestones, echinoderm plates commonly have syntaxial calcite overgrowths. In polarized light and crossed nicols, both the overgrowth and original grain move into extinction together.
Systematic errors: Errors that are predictable and repeating and mostly correctable. This includes errors in instrument measurements, usually quoted as + or – values, or plotted as error bars. Cf Random errors.
Tetragonal crystal system: Liken this group to isometric crystals stretched along the c axis.
a1 = a2 ≠ c α = β = γ = 90o Mostly 2- and 4-fold symmetry. Common crystal forms: Prisms, bipyramids with or without prisms. e.g., zircon, chalcopyrite, rutile
Thermal equator: The latitude where Earth’s surface temperatures are a maximum. It is currently about 10o north of the geographic equator, because the northern hemisphere averages 1.2o to 1.5o higher than the southern hemisphere. There is some seasonal variation in its location. There have been major latitudinal shifts in its position over the past 100,000 years, that have led to significant, frequently abrupt climate change.
Thermocline: The ocean layer extending from about 200m to 1000m depth where the temperature decreases rapidly. Below the thermocline the water temperature varies little from about 4o
Thermodynamics: The investigation of heat, mechanical and chemical work, and energy in any system that moves from one state to another state, and their relationship to energy and entropy. State functions like entropy, enthalpy, and Gibbs Free Energy are usually expressed in terms of temperature, pressure, volume, and mass. The behaviour of these functions is governed by the three classical Laws of Thermodynamics; A fourth law, the Zeroth Law has also been proposed (see under entries for 1st, 2nd, 3rd, and Zeroth laws). Classical Thermodynamics generally refers to the properties of macroscopic systems (e.g. engines, groundwater flow). Statistical thermodynamics generally refers to microscopic or atomic-molecular scale processes.
Third Law of Thermodynamics: This Law, first derived in 1906 by Walter Nernst, is concerned with the behaviour of materials at temperatures approaching absolute zero (0o K, -273oC, -459oF). The guts of the law states that a perfect crystal at 0oK will have zero entropy. The crystal must not have any defects because this would create some disorder and therefore a positive value of entropy. At absolute zero there is no heat – basically everything comes to a standstill. Theoretically this is impossible. The concept of absolute zero and zero entropy go hand in hand. From a quantitative perspective this is useful because it permits an absolute scale for entropy as a function of absolute temperature. Which gives rise to the principle that entropy increases with increasing temperature.
Thermogenic hydrocarbons: Natural oils and gases produced by the breakdown of organic matter at temperatures usually >60o C, and most prolifically between 80o – 120o C. Depending on the geothermal gradient, these temperatures commonly correspond to sediment burial depths of 2-4 km.
Triclinic crystal system: The least symmetric group. a ≠ b ≠ c α ≠ β ≠ γ ≠ 90o
No axes of symmetry! Common crystal forms: Prisms, bipyramids. e.g., microcline, plagioclase, kyanite
Triple point: On a phase diagram, it is the point in pressure-temperature space where solid, liquid and gas phases of a compound coexist.
Tufa: A natural, surface precipitate of calcium carbonate in alkaline lakes, rivers, springs and geothermal hot pools, promoted by degassing of CO2 as the waters exit to the surface. Degassing of CO2 results in an increase in pH, and concomitant increase in the stability of CO32- and HCO3– aqueous species and the degree of calcite saturation. It is also possible that microbial activity also promotes precipitation. Tufas tend to be highly porous; they can encase dead critters and vegetation. Travertines are a denser form of surface calcite precipitation. Extensive deposits are typically terraced.
Twinning: A symmetrical intergrowth of two separate crystals of the same mineral, that share the same mineral lattice. In thin section under crossed nicols, each twin segment will go into extinction at different rotations of the microscope stage. There are many kinds of twinning. For example, plagioclase may show albite, carlsbad, or pericline twins individually or as combinations in the same crystal. Important optical properties of twins that help mineral identification include extinction angle (whether straight or inclined), and 2V angles. Note that twin planes are NOT the same as planes of crystal symmetry.
Unit cell: At the atomic scale, the arrangement of atoms that represents the fundamental structure of a mineral in crystal form. The crystals we see consist of a three-dimensional array of stacked unit cells. This means that the overall shape of the crystal mimics its unit cell. The simplest unit cell is a cube; cubes of the same size will stack perfectly. Not all polygonal geometries allow such stacking, for example cells with triangular sides will stack neatly together, but those with 5-sided faces (pentagons) will not. Consideration of the unit cells and their symmetry forms the basis for definition of the 6 (or 7) crystal systems.
Unsaturated condition: A solution in which the concentration of a dissolved compound is less than saturation, such that the ratio of the measured ion activity (or concentration) product and the standard solubility product (Ksp) is <1.
Vadose zone: The portion of an unconfined aquifer above the watertable where pore spaces are air-filled (and approximately at atmospheric pressure). It is synonymous with unsaturated zone.
Valence: The number of electrons of an ion shared with other ions. The Carbon in CH4 has a valence of 4; each H has a valence of 1.
Vitrinite reflectance: Vitrinite is a component of coal that forms by thermal alteration of plant tissues. The intensity of reflection from a polished surface of vitrinite samples increases with coal rank. The reflectance is measured and compared with standard values d to determine coal rank.
Weak acid: A general classification that depends on how easily an acid donates a proton (H+ ) to a water molecule to form H3O+ . A weak acid will partially dissociate (i.e. split into its constituent H+ and anion, leaving some of the acid in solution. All the reactions involving carbonate and carbonic acid are weak acid reactions. cf. strong acids
Weight: Weight is the force of gravity acting on an object; it has the units of force (SI unit is the Newton), and the same dimensions – MLT-2. Weight is calculated from Newton’s Second Law that is expressed as force F = Mass x Acceleration – in this case acceleration due to gravity. Weight ≠ Mass. For example, a 1 Kg mass on Earth weighs 9.8 N; on the Moon the same mass weighs 1.62 N (Lunar value of g is 1.62 m.s-2, and on Jupiter it weighs 24.79 N where g = 24.79 m.s-2.
Zeroth Law of Thermodynamics: It states that if two thermodynamic systems are both in thermal equilibrium with a third system, then the two systems are in thermal equilibrium with each other. i.e. systems that are in thermodynamic equilibrium will not exchange heat. The Law provides an independent definition of temperature without reference to entropy (as in the 2nd Law).