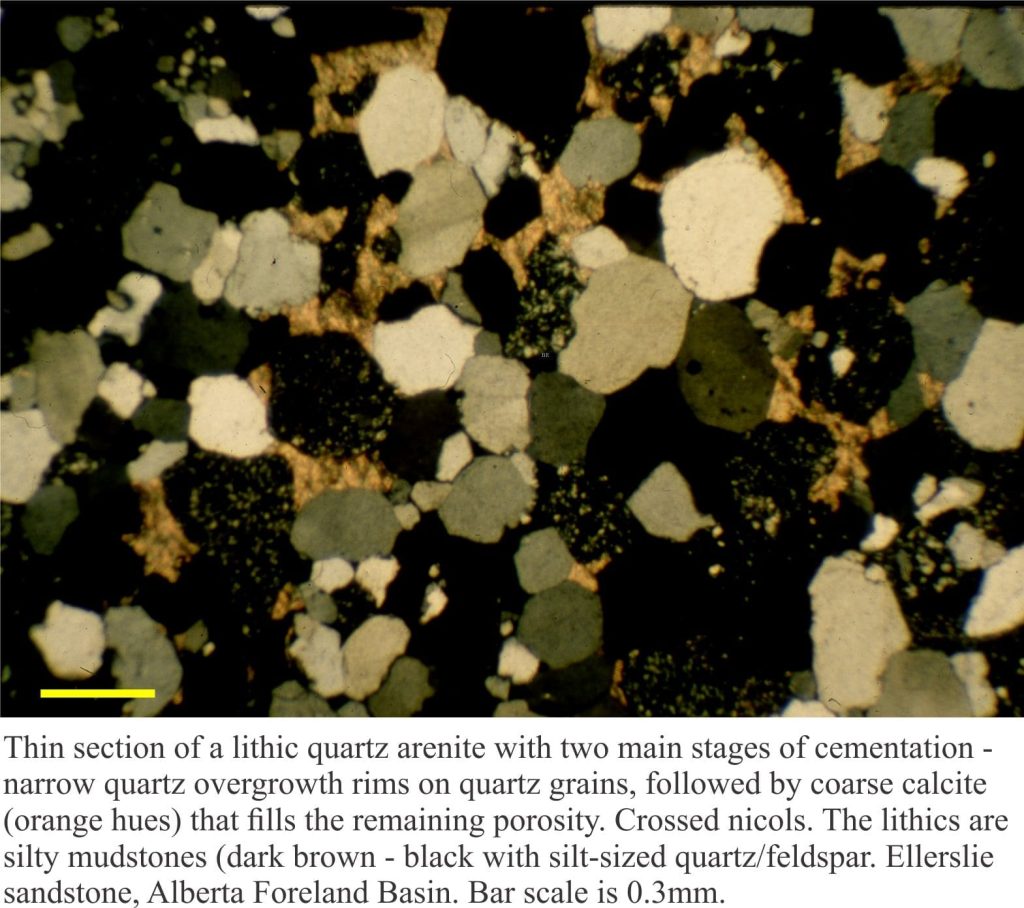
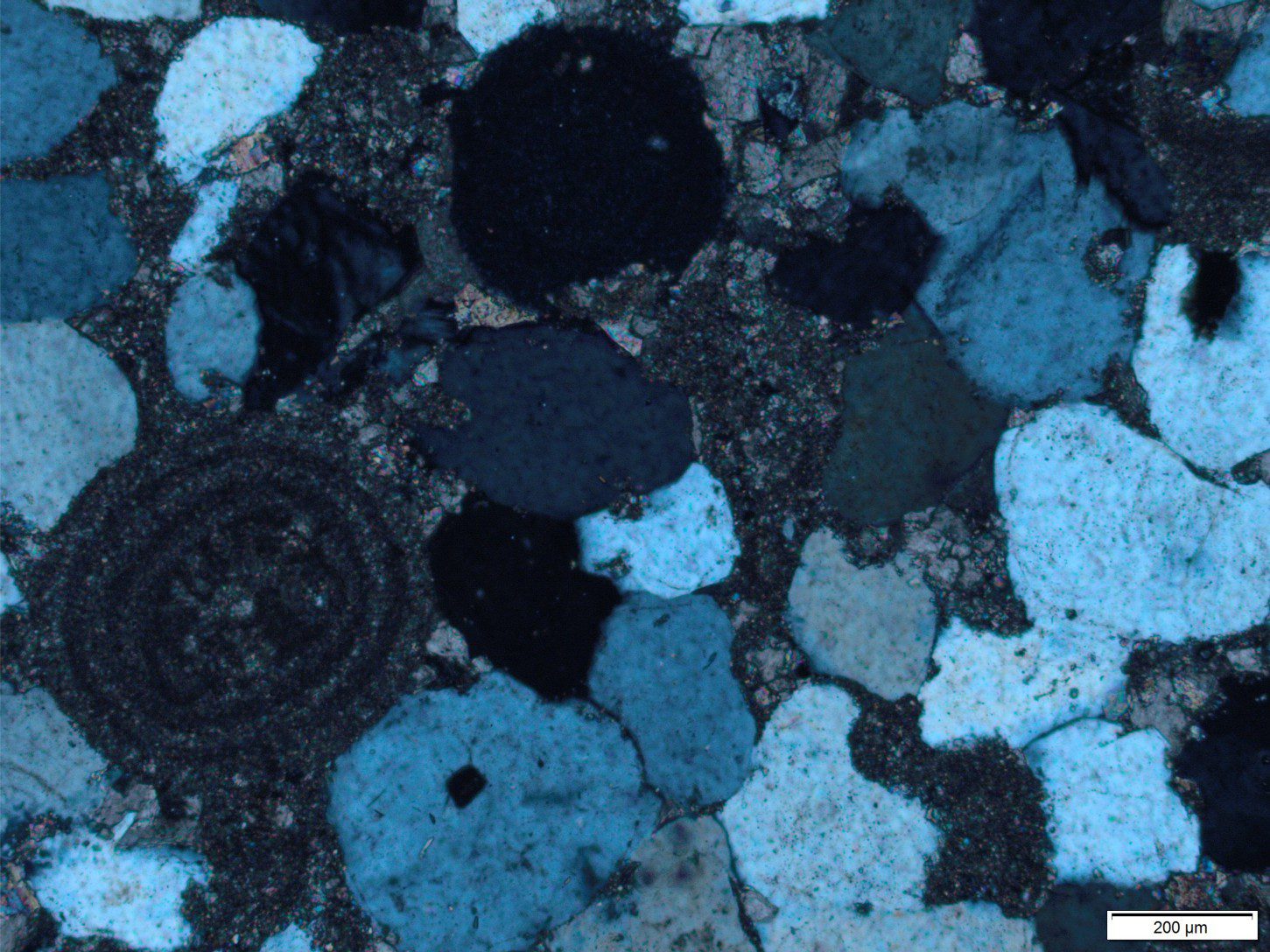
This post is part of the How To… series – How to identify matrix and cement in arenites
The primary architectural element of a sandstone is its framework of sand grains. Spaces between grains will be filled, to varying degrees by much finer sediment – or matrix. The amount of matrix initially deposited is strongly dependent on the energy of the depositional system and the degree of sediment reworking. Wind-blown dune sands and beach sands will have very little matrix at the time of deposition; those deposited farther out to sea will tend to accumulate more.
The proportion of matrix to framework is an important determinant of sandstone classification. Arenites have less than 15% matrix, wackes have more than 15%. Sandstones with little matrix (commonly referred to as clean sands) have the potential to preserve some of their original porosity.
Detrital matrix generally consists of clay minerals mixed with silt-sized quartz and feldspar. At the point of deposition there is a significant volume of interstitial water, most commonly seawater or freshwater. The mix of solid and liquid phases means that matrix is highly reactive in both mechanical and chemical contexts. Compaction that begins soon after deposition and continues during burial, will physically compress the matrix and at the same time drive off some of the interstitial fluid. As burial proceeds, the increase in temperature and accompanying fluid flow will promote chemical reactions involving dissolution of some detrital minerals (particularly clays and feldspar) and precipitation of new minerals. Thus, the matrix gradually changes from purely detrital to a mix of detrital components and diagenetic products. At some point in this transformation there may be no recognisable detrital matrix.
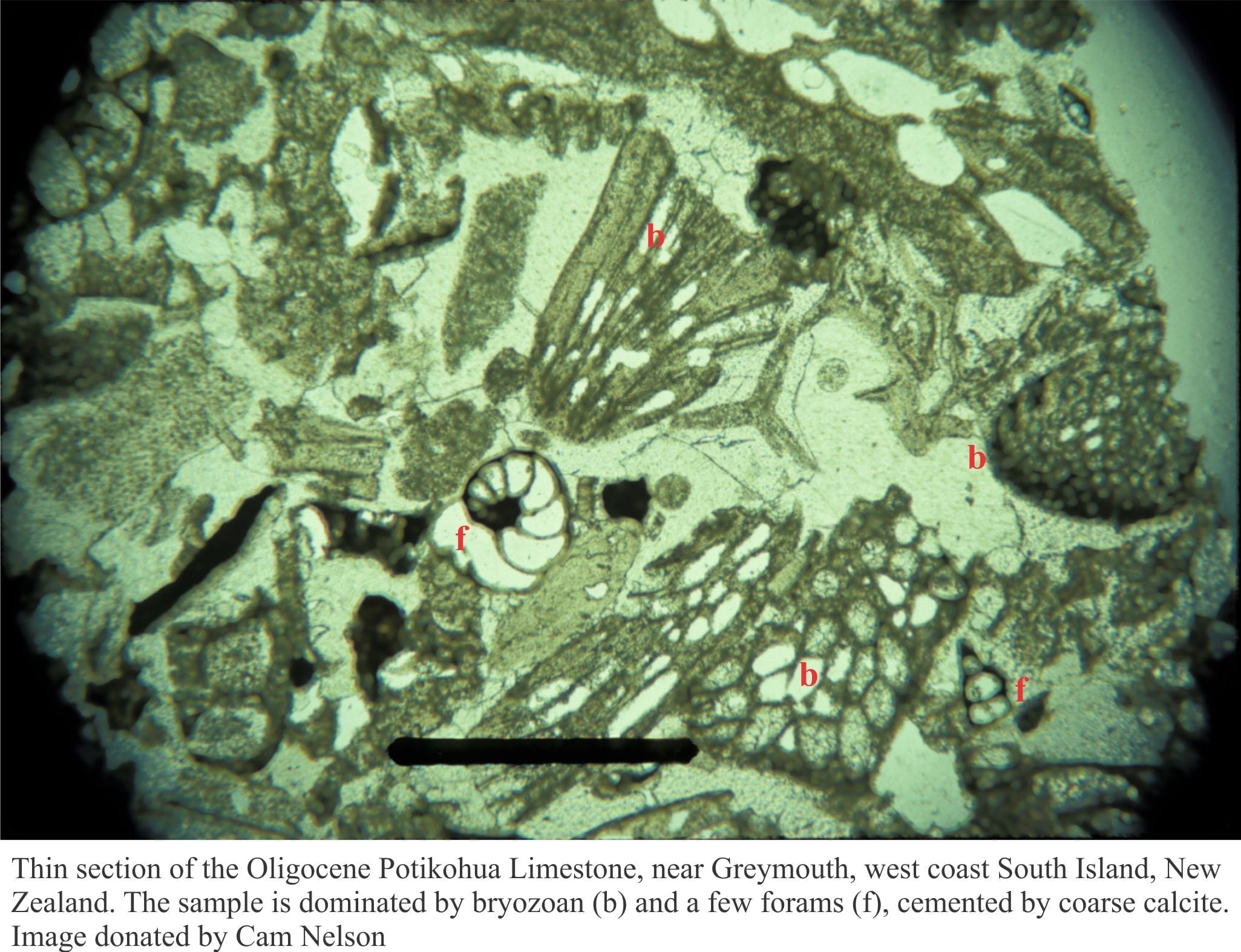
“The greywacke problem” exemplifies the challenges of distinguishing detrital matrix from diagenetically altered matrix. Greywackes are indurated sandstones characterised by high proportions of matrix. They are commonly, but not exclusively associated with turbidite successions. Greywackes tend to be poorly sorted with frameworks of quartz, feldspar and lithics that, in terms of grain size, commonly merge with matrix. The “problem” arises with lithic fragments that are chemically reactive. With diagenetic alteration, the lithics become almost indistinguishable from the surrounding matrix. It is now generally accepted that greywacke matrix consists of alteration products like clays, chlorite, white micas and some calcite. Continued burial and induration will eventually result in the formation of minerals like zeolite and prehnite at low metamorphic grades, and muscovite-biotite at higher levels of metamorphism.
W.A. Cummins 1962 paper “The greywacke problem” is a classic contribution to this discussion.
Cements are post-depositional diagenetic products that fill voids between framework and matrix grains. Cementation can begin soon after deposition (commonly as iron oxides, calcite and aragonite), and continue as the deposits are buried. Cements tend to occlude the original porosity; they may replace matrix and some of the more reactive framework grains. The most common cements in terrigenous sandstones are quartz, calcite, and clays.
Cements may consist of a single mineral or succession of minerals (a kind of cement stratigraphy), depending on the chemical composition and temperature of the fluid that occupies the pores (i.e. interstitial fluid). Fluid flow from pore to pore is also critical – fluid flow will provide a more or less continuous supply of dissolved mass so that cement precipitation can proceed.
Cements composed of a single mineral indicate relatively constant fluid chemistry. Cements composed of more than one mineral indicate evolving fluid chemistry. In either case the cements provide a record of fluid chemistry at the time of precipitation. A good example of cement progression, and one that is quite common in terrigenous sandstones, shows initial precipitation of quartz followed by one or more types of clay (for example kaolinite, illite, or complex mixes with montmorillonite), and the remaining pore space filled by coarse calcite.
Precipitation of quartz commonly takes place as:
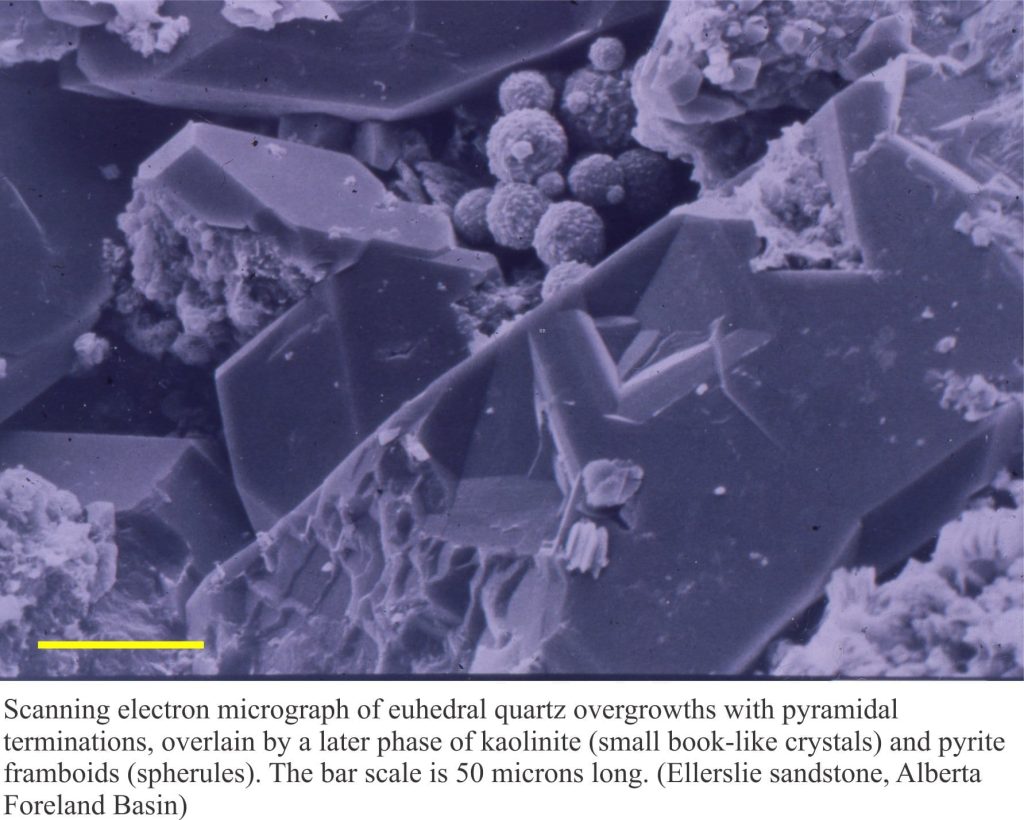
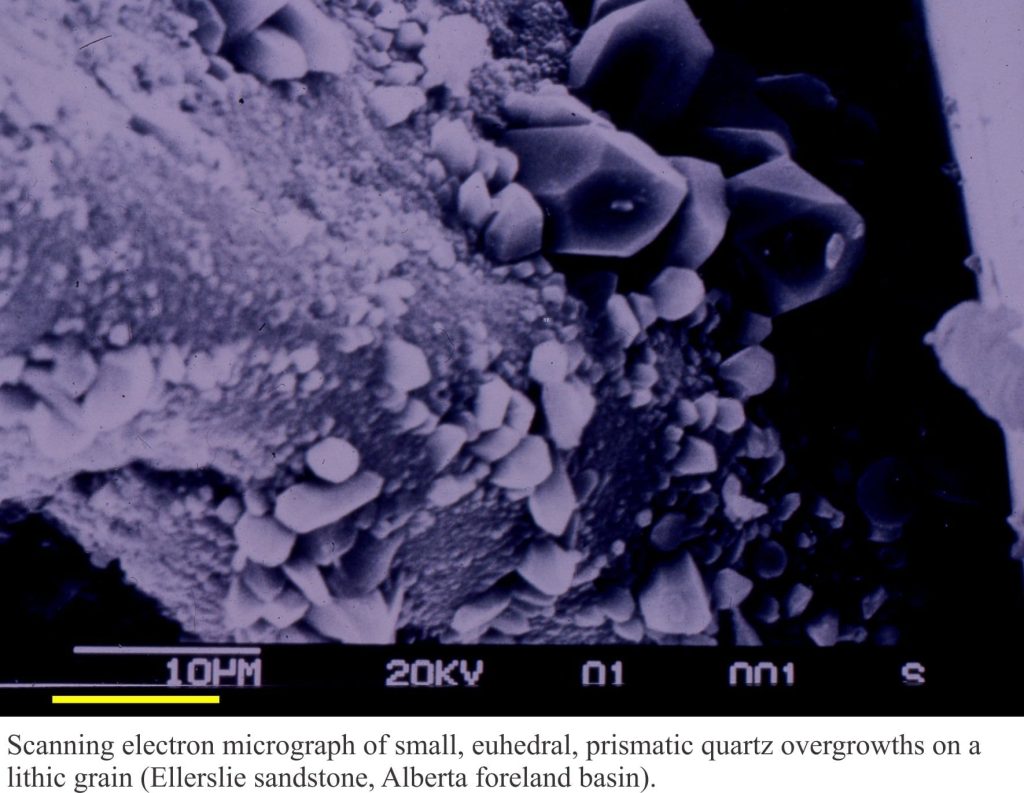
a) Euhedral, prismatic crystals that grow from the surface of lithic, polycrystalline quartz and feldspar grains. Crystal overgrowths like these have no chemical and/or optical relationship with the grain substrate.
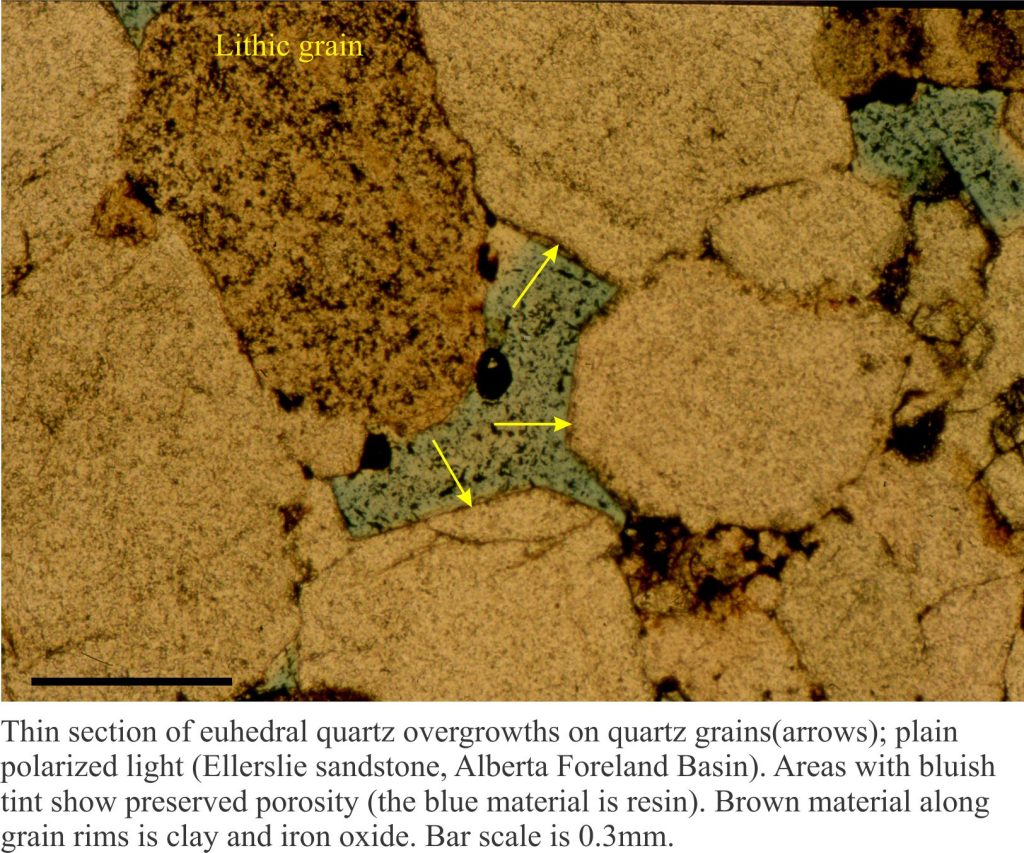
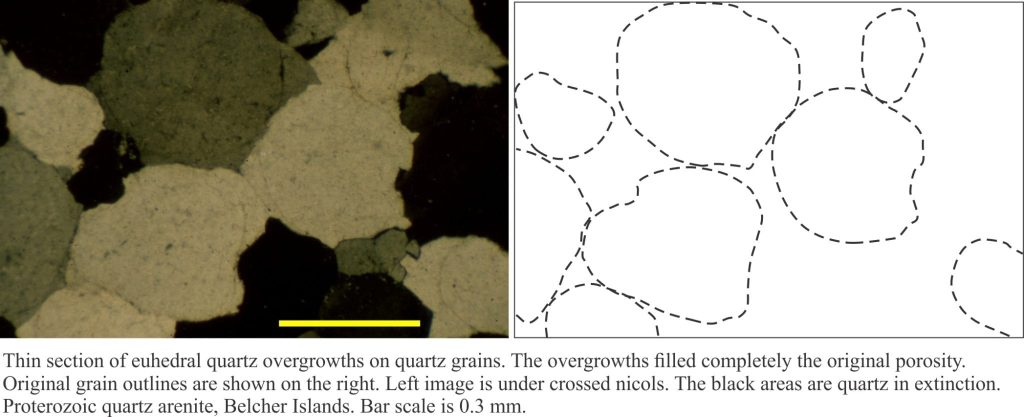
b) Euhedral overgrowths on monocrystalline quartz grains. The overgrowths are typically in optical continuity with the quartz grain – i.e. they share the same crystal axes. These are called syntaxial overgrowths. Overgrowths of this kind are easily identified under crossed nicols because they move into extinction at the same stage as the quartz grain. A rim of inclusions may help to distinguishing the original grain boundary from the crystal overgrowth (check out the images below).
The size of most clay crystals is measured in microns (millionths of a metre) and are difficult to identify with a standard polarizing microscope. One exception is kaolinite that commonly forms mica-like ‘books’ that may be visible at high magnification. Clay minerals are generally identified using a scanning electron microscope and X-ray diffraction.
Under plain polarized light, calcite has significantly higher relief and birefringence than quartz, has excellent cleavage and will usually display a kind of mottled sheen as the microscope stage is rotated. It is common to find calcite replacing quartz and feldspar where contacts between adjacent crystals are embayed. If calcite is a late stage cement, in other words a cement that has overgrown quartz, clays and any other earlier mineral precipitate, it is usually coarsely crystalline. If, on the other hand it precipitated early in a sandstone’s diagenetic history then there may be a progression in crystal size from small rhombohedra that rim the grain surface, to more coarsely crystalline aggregates that fill available pore space. This progression in crystal size is an important indicator of cementation rather than a fabric caused by recrystallization.
The distinction between detrital and diagenetically altered matrix and cements requires careful observation. Don’t rush it! Work systematically across the thin section, zooming on features like grain boundaries and crystal contacts.
Cement history in carbonate rocks is varied and deserves an article on its own!
Some useful links in this series
The mineralogy of sandstones: Quartz grains
The mineralogy of sandstones: Feldspar grains
Describing sedimentary rocks – some basics
Some useful texts:
Petrology of Sedimentary Rocks, Sam Boggs Jr. 2012
Clastic Diagenesis, D. A. McDonald and R. C. Surdam, eds., 1984. AAPG Memoir 37
AAPG Wiki– sandstones