The really ancient earth: How our atmosphere evolved
Take a deep breath. Savour it. One of the few absolutes of our physical world (that we probably haven’t looked after as well as we might have). This post continues the theme “The Really Ancient Earth” by looking at what we know about the origin of our atmosphere; some of the evidence and some of the hypotheses. What was it like on day 1 (about 4600 million years ago) and how did it evolved into our breath-taking world today?
Click on the image to enlarge
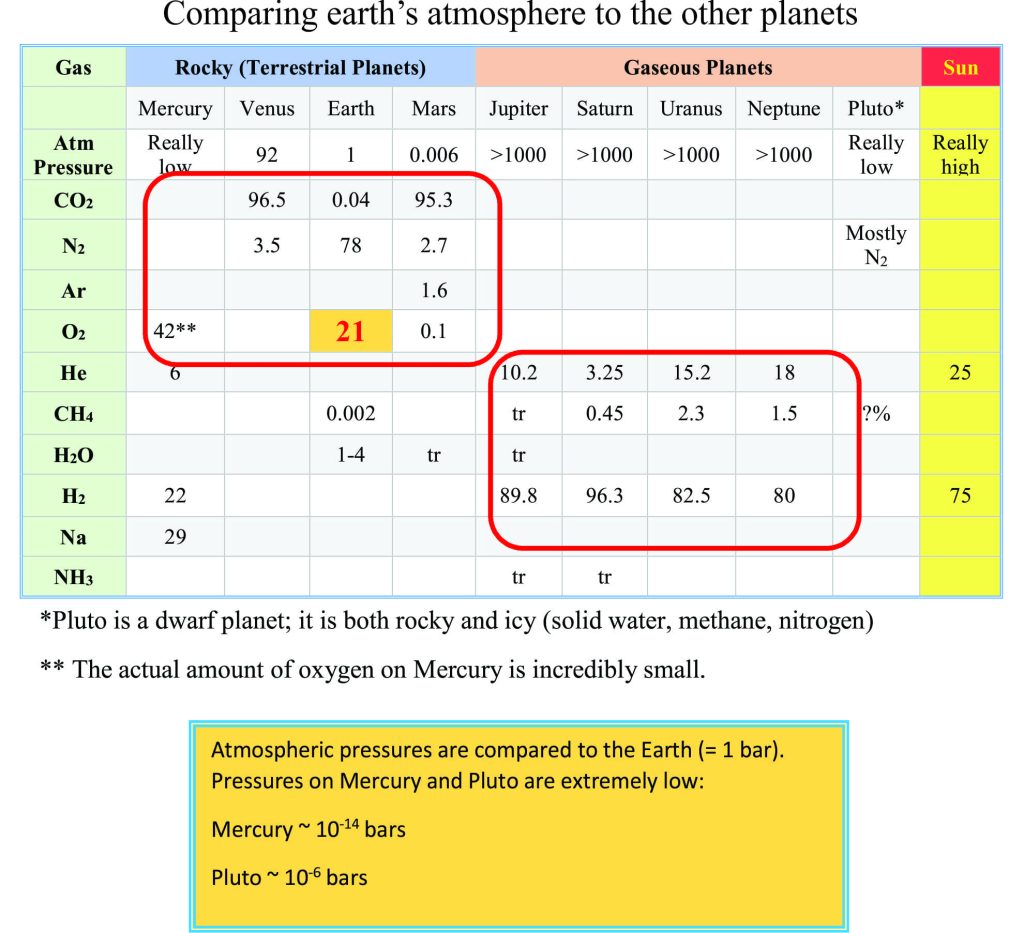
In the grand scheme of things, earth is a rather small rock among several planets, dwarf planets, asteroids, meteors, comets and other space paraphernalia. As the Table below indicates, the percentage of constituents in our atmosphere today is wildly different to all the other planets. We have lots of FREE OXYGEN, but this was not always the case.
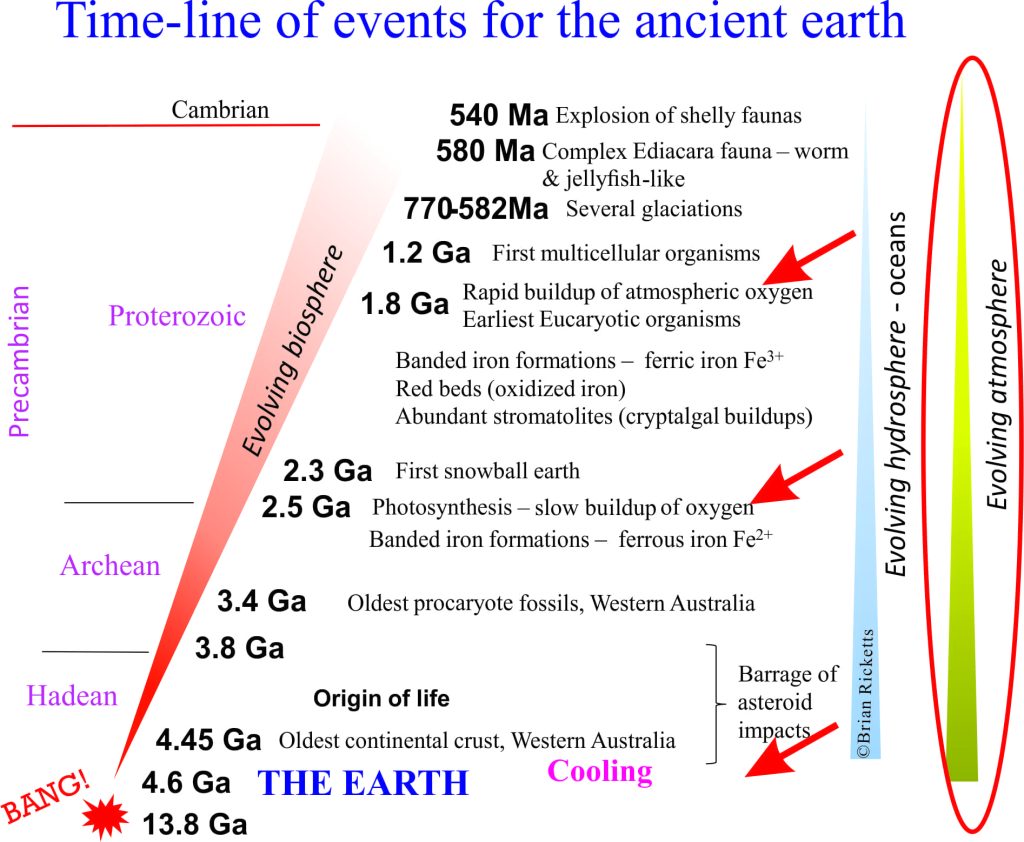
Day 1 (sort of), 4600 million years ago
Our earliest primitive atmosphere probably contained hydrogen (H) and Helium (He), remnants of the solar nebula from which the earth condensed. But most of this escaped, partly because of the initial heat of the newly condensed earth’s crust, and also because of a weak gravity field compared with the other giant gaseous planets (See also a BBC documentary) and a NASA post on Pluto.
So most of the earliest gases, H and He, escaped. What then?
The geological evidence
For decades the prevailing hypothesis determined that the early atmosphere consisted of ammonia (NH3), methane (CH4), carbon dioxide (CO2), hydrogen sulphide (H2S – rotten egg smell); a bit like a pungent, soupy marsh gas. A second school of thought posited volcanic outgassing of Carbon monoxide (CO), carbon dioxide, nitrogen and water.
Recent analysis of very old, stable minerals like Zircon challenge this story. Work at Rensselaer Polytechnic Institute has shown that the magmas that originally produced the zircon crystals were oxidized. Therefore, early magmatic and volcanic outgassing during formation of the primitive crust would have released gases dominated by oxidized compounds, namely CO2, SO2 (sulphur dioxide) and H2O (water), plus Nitrogen (N).
Two other minerals also provide us with clues
Pyrite (FeS2 fool’s gold) and Uraninite (UO2 a natural Uranium mineral) are found as grains and pebbles in ancient sediments. When transported in rivers or shallow seas, these minerals can only survive a short time in Oxygen-rich environments. However, in rocks at least as old as 2400 million years these minerals show no signs of oxidation, indicating that there was very little free Oxygen around at that time.
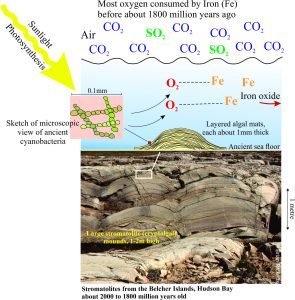
Evidence from stromatolites (cryptalgae, or cyanobacteria)
We also know that at least from about 3400 million years ago there were primitive algae that produced oxygen by photosynthesis. Some oxygen was being produced during these early years, but it was chemically consumed by iron-bearing minerals. We know this because the early Precambrian was witness to the accumulation of vast iron-rich rock formations know as Banded Iron Formations (BIFs) – these are now sources of iron ore in many places). In other words, the iron in the oceans was acting as a chemical SINK that consumed free oxygen to form iron-bearing minerals such as magnetite (Fe3O4).
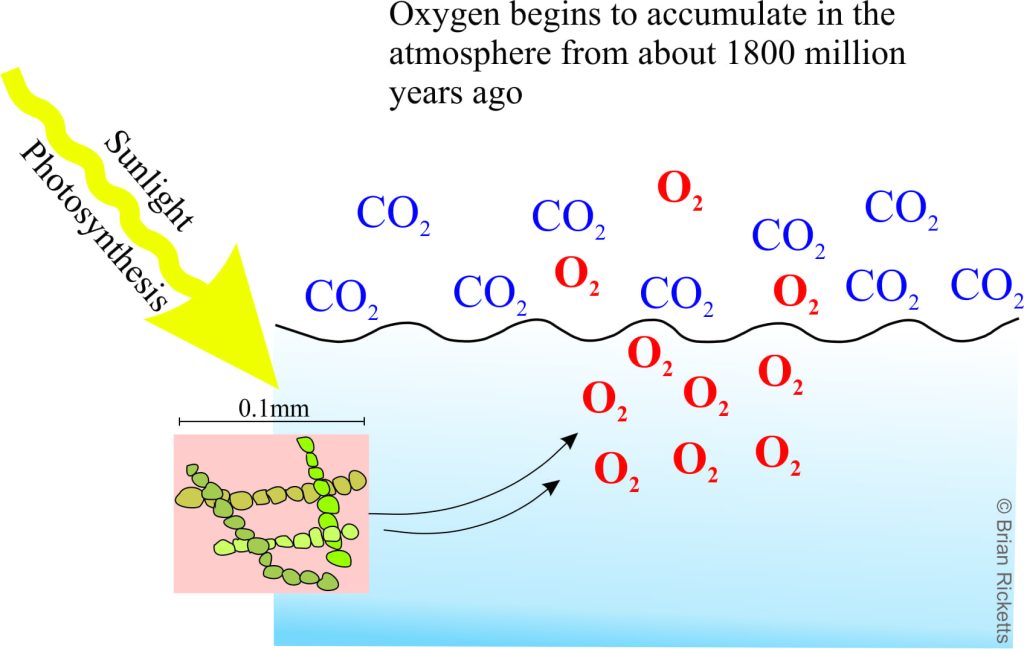
From about 2500 million years and on algal life forms flourished and oxygen production increased. By about 1800 million years ago, the oxygen consumption by iron slowed to the point where it could begin to accumulate in the atmosphere, although CO2 was still the dominant gas (with concentrations many times greater than today). This is commonly referred to as the Great Oxidation Event.
It is also about this time, 1800 million years ago, that another kind of sedimentary rock appears becomes increasingly common – red beds. These are sands and muds deposited mostly on the land surface in rivers or dunes, that have enough iron (in its oxidized state – Fe3+) to give them a distinctive rust-red colour. This is a major turning point in the evolution of the atmosphere. From this time and on, the amount of free oxygen in the atmosphere gradually increased to the point where it could support other, more complex forms of life.
As noted in the first of this series on the Really Ancient Earth, this dramatic, explosive change in the diversity of life forms is heralded by the Cambrian Period, beginning 540 million years ago.
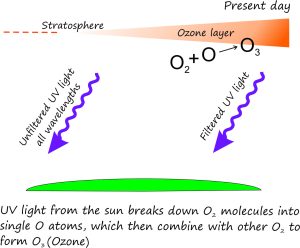
The Ozone layer
Ozone (3 atoms of oxygen) accumulates within the stratosphere where today it filters up to 99% of medium wavelength ultraviolet light (UV). Just as well otherwise we’d be fried!
Prior to about 2500 million years there was little or no free oxygen in the atmosphere – hence no ozone. Conditions for earlier life forms (the ancient algae and bacteria) must have been harsh in a world bombarded by intense UV light. These conditions would have slowly ameliorated as free oxygen accumulated; ozone would also have increased in the stratosphere. Sufficient ozone protection from UV light by the Cambrian (540 million years) may have been one reason for the proliferation of life forms at that time.
The period of geological history before the proliferation of diverse, multicellular life forms (i.e. the Cambrian Period) accounts for about 90% of the total geological history of our earth and solar system. It is an immense span of time. It is sometimes viewed as a rather boring period of earth history, partly because of its remoteness, but also because events such as the production of oxygen took about 2 billion years; 4 times longer than the entire span from Cambrian through to Recent (540 million years). There are still many intriguing and puzzling questions about environmental conditions during the first 4 billion years, about the oceans, the crust and of course life itself. Good science will help unravel at least some of these puzzles.














3 thoughts on “Ancient earth. 3 The air we breath; how our atmosphere evolved”
Thank you so much for this interesting article!. What I wonder,refferring to the pre great oxygenation event, is were the cyanobacteries the only responsible for the production of dioxygene or were there some other factors (biotic or not) that could also free dyoxigene into the water or atmosphere?.
Thanks Pedro. Initially it seems that cyanobacteria were the only microbes, perhaps for the first 1-2 billion years, but from about 2 billion years ago there may have been other microbes – certainly once the Eukaryotes evolved (like red algae) then they too would have contributed O2. Abiotic mechanisms for producing O2, such as lightning, electrolysis etc. are pretty inefficient, at least for creating a whole world of oxygen. Photosynthesis is the most efficient way to produce an atmosphere of oxygen.
Thank you!, it is really helpful.