CO2 has a bad rep. We can’t do without it (GOOD – it’s part of the photosynthetic process), but it looks like we’re upsetting the balance between having too little and producing too much (BAD). I take some of the blame for this: I drive a car (out of necessity), run a small boat (that I really enjoy), use a gas stove (the best cooking device ever), use a couple of lawn/orchard mowers (also necessary to keep the weeds at bay in our organic kiwifruit orchard), and take trips to Canada and beyond (which is life-affirming). I guess we all have our crosses to bear (INDIFFERENT), but I do take solace in the knowledge that my carbon footprint is more than offset by the biomass on my organic orchard.
Back to geology
Carbon moves through a grand cycle. The cycle is actually pretty complex and if considered in its entirety, takes 10s of millions of years wherein carbon moves through the atmosphere (mainly as carbon dioxide and methane), the biosphere (that includes us), the earth’s crust and mantle (i.e. soils, rocks, magma), and the oceans. The exchange of carbon through these systems can take place fairly quickly even on a human timescale, or it can take eons.
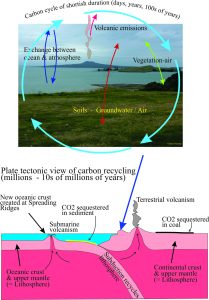 Climate change science tends to concentrate on the atmosphere-ocean part of the carbon cycle, probably because it is the most obvious and also because it is the most likely to influence our well-being in the short term (a few lifetimes). However, important parts of the carbon cycle also include processes that can take a very long time, such as the recycling of carbon, rock and fluid through the crust and mantle over 10s of millions of years, only to be rejuvenated when new crust is formed, for example by volcanoes. Carbon is also stored, or sequestered as rock and fluid and it is the use of some of these (coal, oil, gas) that has the potential to disrupt the natural cycle.
Climate change science tends to concentrate on the atmosphere-ocean part of the carbon cycle, probably because it is the most obvious and also because it is the most likely to influence our well-being in the short term (a few lifetimes). However, important parts of the carbon cycle also include processes that can take a very long time, such as the recycling of carbon, rock and fluid through the crust and mantle over 10s of millions of years, only to be rejuvenated when new crust is formed, for example by volcanoes. Carbon is also stored, or sequestered as rock and fluid and it is the use of some of these (coal, oil, gas) that has the potential to disrupt the natural cycle.
Ocean – Atmosphere exchange
CO2 is absorbed quickly in water. You can see this in your soda-stream maker or can of fizzy pop. When you open a bottle of soda water CO2 is released as bubbles. If you put the bottle cap back on the bubbles will continue for a while but gradually decrease until all bubbling ceases. This is the point at which the CO2 pressure in the air space is in equilibrium with that in the water. This doesn’t mean that there is no exchange of CO2 between the air and water in the bottle, but that the amount of CO2 going in or out is the same; there is an equilibrium, or balance.
Exchange between the oceans and atmosphere accounts for about 43% of CO2 in the air. However, we need to remember that the exchange is not a one-way journey. There is an approximate equilibrium between the two sources of CO2 – the atmosphere and the oceans, that means there is as much CO2 going into the atmosphere as there is CO2 going into the oceans. This balance can be upset, but even when this happens the tendency is for the atmosphere-ocean system to establish a new equilibrium.
It is estimated that 36 giga (billion) tons of CO2 were released into the atmosphere in 2013. The oceans are capable of absorbing massive amounts of CO2 but there are limits; many scientists now think those limits have been reached. This may well be the case, but we do need to take stock of some other factors in the ocean-atmosphere exchange story.
- When CO2 exchanges with seawater, some of it remains as CO2 in water (it is absorbed), and some of it dissolves forming the following products during chemical reactions: H2CO3 (carbonic acid – a weak acid), HCO3 (bicarbonate), CO3 (carbonate), H+ (acid). Carbon is removed from seawater itself when calcite and aragonite precipitate (as shells, skeletons and so on). Under normal conditions there is a balance between these chemical components such that in seawater the index of acidity, the pH is maintained at about 8.2 i.e. slightly basic (the neutral acid-base value is 7). In other words, the chemistry of carbon in seawater acts to buffer (maintain) the pH.
- CO2 is absorbed or dissolved into ocean surface waters. It takes time for these waters to mix with deeper oceanic water masses. In fact, some deep oceanic circulation systems can take several 100 years to move to shallower depths. This means that there are some pretty old deep waters that may contain pre-industrialization concentrations of CO2. So for example, if a process like ocean acidification is taking place it will not affect all shallow and deep ocean water masses at the same time. How long it might take to ‘homogenise’ ocean pH is not well known.
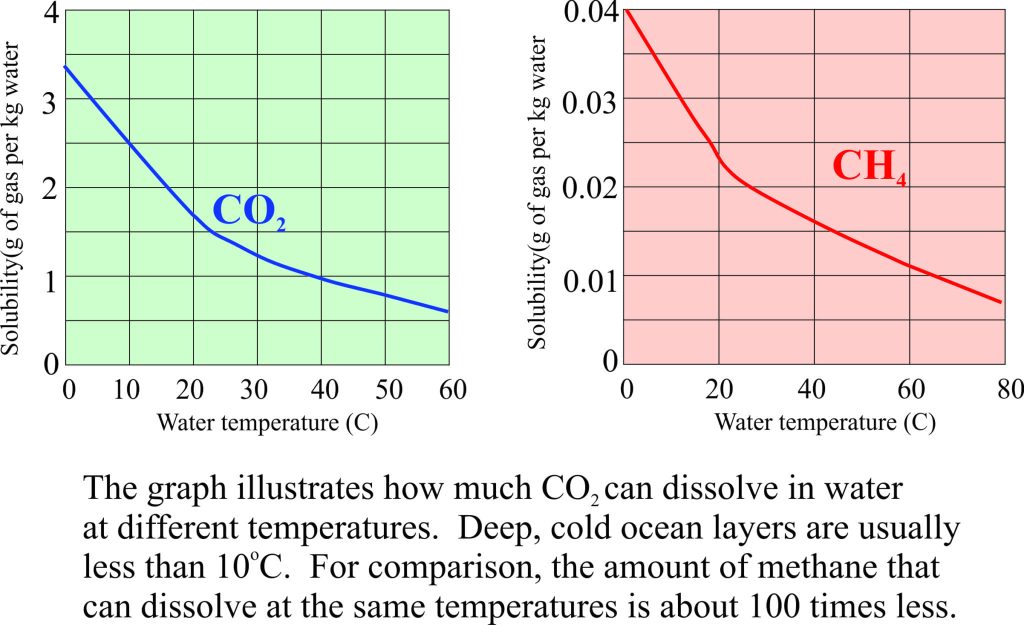 Cold water will absorb more CO2 than warm water. As ice sheets melt, the cold water outflow will tend to have higher CO2 concentration than warmer waters, especially those in the tropics. The geographic distribution of these differing CO2 concentrations will depend on ocean currents and ocean mixing. The short and longer term impacts of mixing on ocean acidity are not that well understood at present although some evidence has been presented for a slight lowering of pH in shallow Great Barrier Reef waters.
Cold water will absorb more CO2 than warm water. As ice sheets melt, the cold water outflow will tend to have higher CO2 concentration than warmer waters, especially those in the tropics. The geographic distribution of these differing CO2 concentrations will depend on ocean currents and ocean mixing. The short and longer term impacts of mixing on ocean acidity are not that well understood at present although some evidence has been presented for a slight lowering of pH in shallow Great Barrier Reef waters.
Volcanoes; The Recycling Depots
CO2 is a common component of volcanic gasses. The US Geological Survey has calculated that about 200 million tonnes of CO2 are contributed annually by land and submarine volcanoes. The CO2 emitted by volcanoes is dissolved in magmas deep in the earth’s crust and mantle. Like the soda-stream bottle experiment, the gas is released from the magma as it rises to the surface and the gas pressure decreases. The most prominent submarine volcanic systems are the mid-ocean ridges, or spreading ridges where new crust is formed by rising magma. Large volumes of CO2 are released there into the ocean waters. Some of this CO2 will also react chemically with the new rocks produced by the rising magma and these CO2 + Water + Rock reactions will tend to remove carbon from seawater.
The volume of CO2 calculated by the USGS may be underestimated because it has also been discovered that CO2 is also released through faults associated with these large, linear rifts and not just the volcanic vents themselves. Nevertheless, the amount released by volcanic processes, even if underestimated by a factor of 2 or 3, is only a small fraction of the total CO2 added by burning fossil fuels.
Natural Sequestration
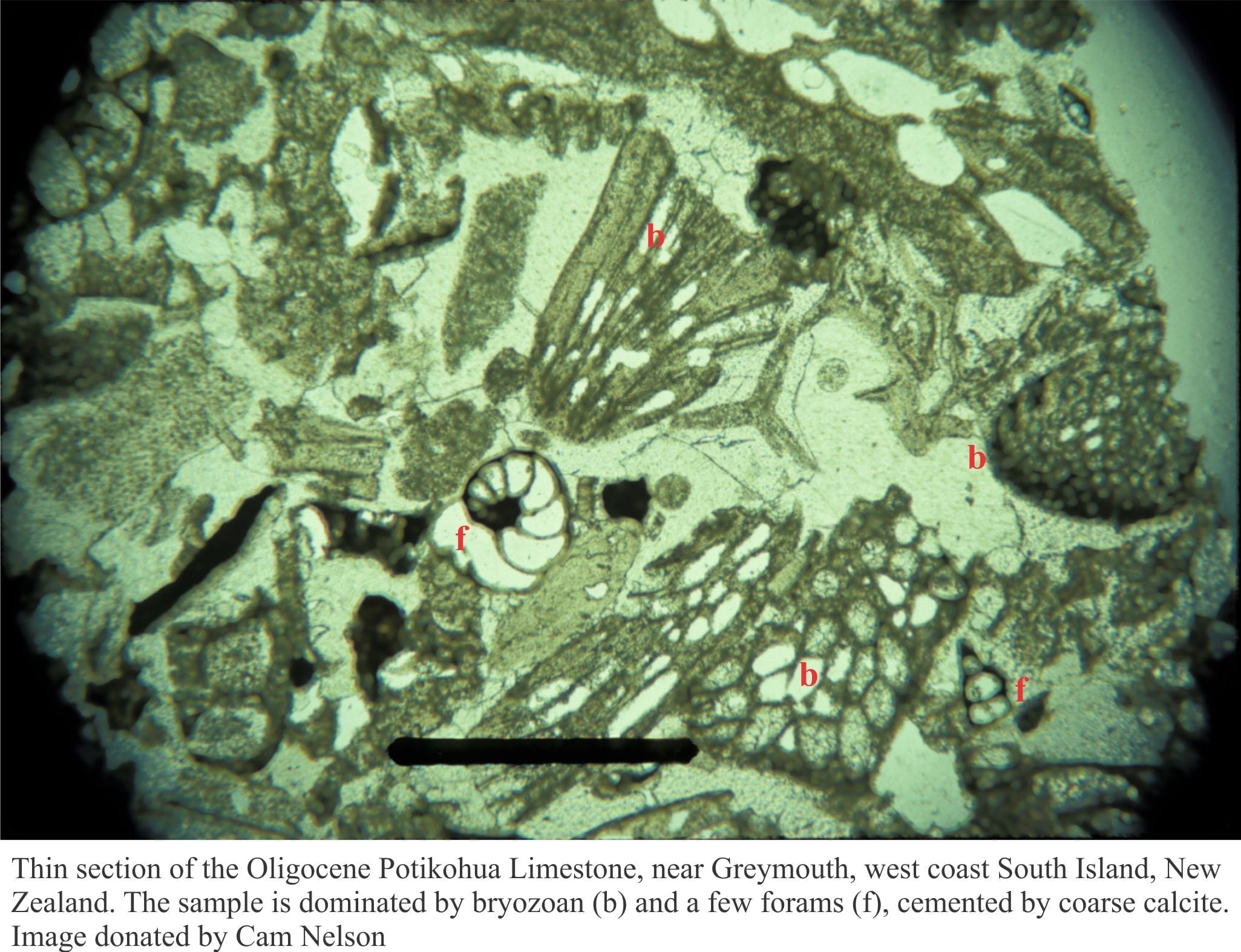
 A natural ‘sink’ for carbon and CO2 in the oceans is the formation of calcium carbonate as either the mineral calcite or aragonite, that is precipitated by organisms such as plankton, certain types of algae, shellfish, corals, bryozoans and crawling-swimming critters. When calcite or aragonite forms, the chemical reactions remove CO2 from seawater.
A natural ‘sink’ for carbon and CO2 in the oceans is the formation of calcium carbonate as either the mineral calcite or aragonite, that is precipitated by organisms such as plankton, certain types of algae, shellfish, corals, bryozoans and crawling-swimming critters. When calcite or aragonite forms, the chemical reactions remove CO2 from seawater.
Over long periods of time the dead shell-skeletal material accumulates on the sea floor, is buried and hardens to limestone. Most of the limestones we see today are acting as vast storage facilities for carbon – they have sequestered carbon.
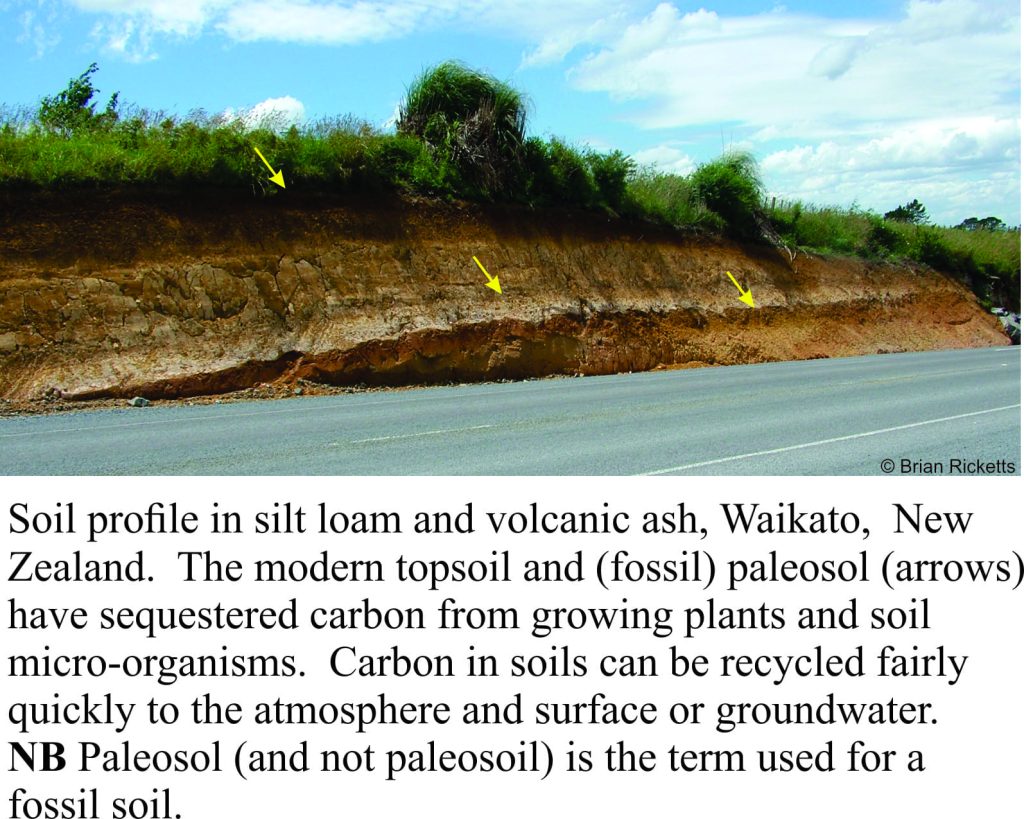
An analogous process takes place with land plants that accumulated in bogs and swamps, were buried and converted over millions of years to coal. Hydrocarbons (oil and gas) also represent the long-term sequestering of organic carbon that, through geological history will have been present in the ancient atmosphere, oceans and organisms. At a quicker pace, modern soils sequester carbon that is used both by plants and micro-organisms.
Permafrost – more tenuous sequestration
Arctic permafrost covers about 24% of the northern hemisphere land area; it also extend beneath the sea floor. Permafrost consists of ice, frozen soil and sediment. Although some CO2 is present in the ice, most permafrost carbon is locked in frozen organic matter. Melting has the potential to release carbon; some will be consumed by soil microbes and plants, and some will be released as CO2 and methane CH4, depending on the metabolism of the microbes. Groundwater chemistry may also change as CO2 and ions like calcium or sulphate are released. Indeed, the chemistry of Arctic drainage systems like Yukon and MacKenzie rivers seem to be undergoing some changes to their chemistry as a result of permafrost melt.
Afterword
Debates over climate change, whether they are directed towards agreement or denial, encapsulate positions that at one end of the spectrum are ideological and political, and at the other scientific. Ideological positions, regardless of stripe, contribute little to the debate other than providing a vent for one’s spleen. Ultimately, questions about the veracity of the science will be answered by science.
The USGS has calculated that, in 2013, 36 giga tons of CO2 were added to the atmosphere-ocean system; this is a humongous volume. It seems to dwarf the natural CO2 inputs from volcanoes, soils, organisms and so on.
The problem, if we encapsulate it in a single sentence, is that the rate of CO2 emissions into the atmosphere-ocean system is far greater than the rate at which CO2 can be consumed or sequestered by all natural processes. Does this mean that climate will change? Regardless of one’s position on Climate Change it seems eminently sensible to allow the science to continue gathering data that will test the veracity of hypothesis and theory. Afterall, many-most of us will have grandchildren and I for one would like to be more certain about the kind of world I’m leaving them.











3 thoughts on “CO2 – the Good, the Bad, and the Indifferent”
QUESTION:
What would be the most effective method to persuade those who doesn’t believe in the existence of climate change?
ANSWER:
Step 1. Show the evolution of the CO2 content in the atmosphere in the last 800,000 years (e.g. http://www.esrl.noaa.gov/gmd/ccgg/trends/history.html). Underline that there is a correlation between global CO2 content in the atmosphere and climate (http://www.ncdc.noaa.gov/paleo/globalwarming/temperature-change.html ). Climate change occurred in the past, so that the existence of climate change on its own should be out of question.
Step 2. Note that not even during the interglacial (hot) periods CO2 exceeded 300 ppm (worldwide average), but after the start of the so-called industrial revolution (beginning of massive use of fossil fuels, coal, oil, gas) the CO2 level started to rocket and reached 400 ppm (it grows now at a rate of approx. 2 ppm/year). The point becomes finding what causes such an anomalous growth of CO2 concentration in the atmosphere (which is likely to bring climate change if not cured).
Step 3. Observe two years of CO2 man-made emissions (animation on page http://www.esrl.noaa.gov/gmd/ccgg/trends/ff.html) and reflect on the fact that fossil fuels are relatively poor in 13C, a stable isotope of Carbon. By burning 13C depleted fuel, people generates 13C depleted CO2 that mixes in the atmosphere (initially in the same hemisphere where fossil fuel is burned). Observe how the 13C content of atmospheric CO2 diminishes in time (http://www.esrl.noaa.gov/gmd/ccgg/gallery/figures/co2c13_surface.png ) with an obvious correlation to the CO2 content (http://www.esrl.noaa.gov/gmd/ccgg/gallery/figures/co2_surface.png), proving that the excess CO2 is man-made (http://www.esrl.noaa.gov/gmd/outreach/isotopes/c13tellsus.html , and http://www.esrl.noaa.gov/gmd/outreach/isotopes/mixing.html).
Step 4. Put all together and conclude that A) mankind is emitting to the atmosphere CO2 from burning fossil fuels at a rate that exceeds the natural capacity of the planet to take it back, and that B) such excessive CO2 is likely to cause severe climate changes (if no remedies are taken).
Step 5 (optional, but strongly recommended). Start campaigning in favour of CO2 emission reduction, including Carbon Capture and Storage (CCS, see for example http://www.globalccsinstitute.com/).
Not to mention the research that shows more and more carbon being released from soils as the global temperature rises. So there is another long term factor that also effects the shorter term factors.
Soil carbon is pretty complex. Most taken up by plants and the microbiota, especially the microbiota. Some is released to the atmosphere but it is v difficult to generalise about how much, where and when.